New functions of XPC in the protection of human skin cells from oxidative damage
- PMID: 16957781
- PMCID: PMC1570445
- DOI: 10.1038/sj.emboj.7601277
New functions of XPC in the protection of human skin cells from oxidative damage
Abstract
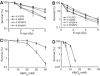
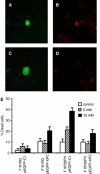
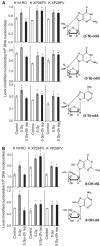
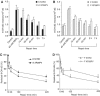
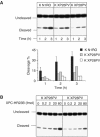
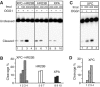
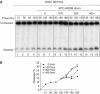
Xeroderma pigmentosum (XP) C is involved in the recognition of a variety of bulky DNA-distorting lesions in nucleotide excision repair. Here, we show that XPC plays an unexpected and multifaceted role in cell protection from oxidative DNA damage. XP-C primary keratinocytes and fibroblasts are hypersensitive to the killing effects of DNA-oxidizing agents and this effect is reverted by expression of wild-type XPC. Upon oxidant exposure, XP-C primary keratinocytes and fibroblasts accumulate 8,5'-cyclopurine 2'-deoxynucleosides in their DNA, indicating that XPC is involved in their removal. In the absence of XPC, a decrease in the repair rate of 8-hydroxyguanine (8-OH-Gua) is also observed. We demonstrate that XPC-HR23B complex acts as cofactor in base excision repair of 8-OH-Gua, by stimulating the activity of its specific DNA glycosylase OGG1. In vitro experiments suggest that the mechanism involved is a combination of increased loading and turnover of OGG1 by XPC-HR23B complex. The accumulation of endogenous oxidative DNA damage might contribute to increased skin cancer risk and account for internal cancers reported for XP-C patients.
Figures


References
-
- Araki M, Masutani C, Takemura M, Uchida A, Sugasawa K, Kondoh J, Ohkuma Y, Hanaoka F (2001) Centrosome protein centrin 2/caltractin 1 is part of the xeroderma pigmentosum group C complex that initiates global genome nucleotide excision repair. J Biol Chem 276: 18665–18672 - PubMed
-
- Ballmaier D, Epe B (1995) Oxidative DNA damage induced by potassium bromate under cell-free conditions and in mammalian cells. Carcinogenesis 16: 335–342 - PubMed
-
- Birincioglu M, Jaruga P, Chowdhury G, Rodriguez H, Dizdaroglu M, Gates KS (2003) DNA base damage by the antitumor agent 3-amino-1,2,4-benzotriazine 1,4-dioxide (tirapazamine). J Am Chem Soc 125: 11607–11615 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

