Adeno-associated virus interactions with B23/Nucleophosmin: identification of sub-nucleolar virion regions
- PMID: 16959286
- PMCID: PMC1829415
- DOI: 10.1016/j.virol.2006.07.050
Adeno-associated virus interactions with B23/Nucleophosmin: identification of sub-nucleolar virion regions
Abstract
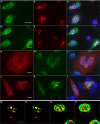
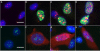
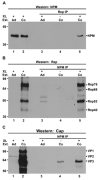
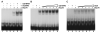
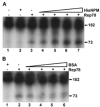
Adeno-associated virus (AAV) is a human parvovirus that normally requires a helper virus such as adenovirus (Ad) for replication. The four replication proteins (Rep78, 68, 52 and 40) encoded by AAV are pleiotropic effectors of virus integration, replication, transcription and virion assembly. Using Rep68 column chromatography and mass spectrometry, we have identified the nucleolar, B23/Nucleophosmin (NPM) protein as an Rep-interacting partner. Rep-NPM interactions were verified by co-immunofluorescence and chemical cross-linking studies. We have found that there is demonstrable, but limited co-localization between Rep and NPM in co-infected cells. In contrast, there was significant co-localization between NPM and AAV Cap proteins. In vitro experiments using purified MBPRep78 and NPM show that NPM stimulates MBPRep78 interactions with the AAV ITR as well as endonuclease activity. These studies suggest that NPM plays a role in AAV amplification affecting Rep function and virion assembly.
Figures
References
-
- Adachi Y, Copeland TD, Hatanaka M, Oroszlan S. Nucleolar targeting signal of Rex protein of human T-cell leukemia virus type I specifically binds to nucleolar shuttle protein B-23. J Biol Chem. 1993;268(19):13930–4. - PubMed
-
- Basrur V, Yang F, Kushimoto T, Higashimoto Y, Yasumoto K, Valencia J, Muller J, Vieira WD, Watabe H, Shabanowitz J, Hearing VJ, Hunt DF, Appella E. Proteomic analysis of early melanosomes: identification of novel melanosomal proteins. J Proteome Res. 2003;2(1):69–79. - PubMed
-
- Borer RA, Lehner CF, Eppenberger HM, Nigg EA. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56(3):379–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

