Mucin granule intraluminal organization
- PMID: 16960124
- PMCID: PMC2176109
- DOI: 10.1165/rcmb.2006-0291TR
Mucin granule intraluminal organization
Abstract
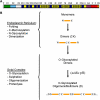
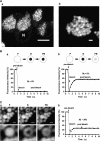
Mucus secretions have played a central role in the evolution of multicellular organisms, enabling adaptation to widely differing environments. In vertebrates, mucus covers and protects the epithelial cells in the respiratory, gastrointestinal, urogenital, visual, and auditory systems, amphibian's epidermis, and the gills in fishes. Deregulation of mucus production and/or composition has important consequences for human health. For example, mucus obstruction of small airways is observed in chronic airway diseases, including chronic obstructive pulmonary disease, asthma, and cystic fibrosis. The major protein component in the mucus is a family of large, disulfide-bonded glycoproteins known as gel-forming mucins. These proteins are accumulated in large, regulated secretory granules (the mucin granules) that occupy most of the apical cytoplasm of specialized cells known as mucous/goblet cells. Since mucin oligomers have contour dimensions larger than the mucin granule average diameter, the question arises how these highly hydrophilic macromolecules are organized within these organelles. I review here the intraluminal organization of the mucin granule in view of our knowledge on the structure, biosynthesis, and biophysical properties of gel-forming mucins, and novel imaging studies in living mucous/goblet cells. The emerging concept is that the mucin granule lumen comprises a partially condensed matrix meshwork embedded in a fluid phase where proteins slowly diffuse.
Figures


References
-
- Perez-Vilar J, Hill RL. The structure and assembly of secreted mucins. J Biol Chem 1999;274:31751–31754. - PubMed
-
- Dekker J, Rossen JW, Buller HA, Einerhand AW. The MUC family: an obituary. Trends Biochem Sci 2002;27:126–131. - PubMed
-
- Fowler J, Vinall L, Swallow D. Polymorphism of the human muc genes. Front Biosci 2001;6:D1207–D1215. - PubMed
-
- Perez-Vilar J, Randell SH, Boucher RC. C-Mannosylation of MUC5AC and MUC5B Cys subdomains. Glycobiology 2004;4:325–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

