Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL)
- PMID: 16960145
- PMCID: PMC1785068
- DOI: 10.1182/blood-2006-06-025999
Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL)
Erratum in
- Blood. 2007 Jun 15;109(12):5086
Abstract
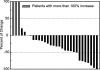
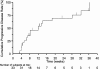
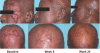
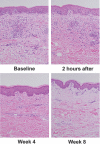
The activity and safety of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid, SAHA) were evaluated in patients with refractory cutaneous T-cell lymphoma (CTCL). Group 1 received vorinostat 400 mg daily, group 2 received vorinostat 300 mg twice daily for 3 days with 4 days rest, and group 3 received vorinostat 300 mg twice daily for 14 days with 7 days rest followed by 200 mg twice daily. Treatment continued until disease progression or intolerable toxicity. The primary objective was to determine the complete and partial response (PR) rate. Time to response (TTR), time to progressive disease (TTP), response duration (DOR), pruritus relief, and safety were determined. Thirty-three patients who had received a median of 5 prior therapies were enrolled. Eight patients achieved a PR, including 7 with advanced disease and 4 with Sézary syndrome. The median TTR, DOR, and TTP for responders were 11.9, 15.1, and 30.2 weeks, respectively. Fourteen of 31 evaluable patients had pruritus relief. The most common drug-related AEs were fatigue, thrombocytopenia, diarrhea, and nausea. The most common grade 3 or 4 drug-related AEs were thrombocytopenia and dehydration. Vorinostat demonstrated activity in heavily pretreated patients with CTCL. The 400 mg daily regimen had the most favorable safety profile and is being further evaluated.
Conflict of interest statement
Conflict-of-interest disclosure: Several of the authors (J.H.C., J.F.R., J.L.R., V.M.R., and S.RF.) have declared a financial interest in Merck & Co., Inc. whose potential product was studied in the present work. One of the authors (J.H.C.) has declared a financial interest in a competitor of Merck & Co., Inc. Several of the authors (J.H.C., J.F.R., J.L.R., V.M.R., and S.R.F.) are employed by Merck & Co., Inc. M.D., as principal investigator, received support from Aton/Merck for conducting the clinical trial and NIH K24 CA86815. The remaining authors (R.T., X.N., C.Z., P.H., and C.K.) declare no competing financial interests.
Figures

References
-
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. - PubMed
-
- Foss F. Mycosis fungoides and the Sezary syndrome. Curr Opin Oncol. 2004;16:421–428. - PubMed
-
- Querfeld C, Rosen ST, Guitart J, Kuzel TM. The spectrum of cutaneous T-cell lymphomas: new insights into biology and therapy. Curr Opin Hematol. 2005;12:273–278. - PubMed
-
- Duvic M, Apisarnthanarax N, Cohen DS, et al. Analysis of long-term outcomes of combined modality therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2003;49:35–49. - PubMed
-
- Duvic M, Hymes K, Heald P, et al. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol. 2001;19:2456–2471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

