The activity of the dinuclear cobalt-beta-lactamase from Bacillus cereus in catalysing the hydrolysis of beta-lactams
- PMID: 16961465
- PMCID: PMC1698674
- DOI: 10.1042/BJ20061002
The activity of the dinuclear cobalt-beta-lactamase from Bacillus cereus in catalysing the hydrolysis of beta-lactams
Abstract
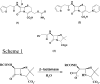
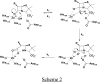
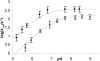
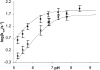
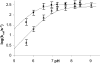
Metallo-beta-lactamases are native zinc enzymes that catalyse the hydrolysis of beta-lactam antibiotics, but are also able to function with cobalt(II) and require one or two metal-ions for catalytic activity. The hydrolysis of cefoxitin, cephaloridine and benzylpenicillin catalysed by CoBcII (cobalt-substituted beta-lactamase from Bacillus cereus) has been studied at different pHs and metal-ion concentrations. An enzyme group of pK(a) 6.52+/-0.1 is found to be required in its deprotonated form for metal-ion binding and catalysis. The species that results from the loss of one cobalt ion from the enzyme has no significant catalytic activity and is thought to be the mononuclear CoBcII. It appears that dinuclear CoBcII is the active form of the enzyme necessary for turnover, while the mononuclear CoBcII is only involved in substrate binding. The cobalt-substituted enzyme is a more efficient catalyst than the native enzyme for the hydrolysis of some beta-lactam antibiotics suggesting that the role of the metal-ion is predominantly to provide the nucleophilic hydroxide, rather than to act as a Lewis acid to polarize the carbonyl group and stabilize the oxyanion tetrahedral intermediate.
Figures



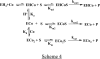
Similar articles
-
Enzyme deactivation due to metal-ion dissociation during turnover of the cobalt-beta-lactamase catalyzed hydrolysis of beta-lactams.Biochemistry. 2006 Sep 12;45(36):11012-20. doi: 10.1021/bi0610146. Biochemistry. 2006. PMID: 16953588
-
The variation of catalytic efficiency of Bacillus cereus metallo-beta-lactamase with different active site metal ions.Biochemistry. 2006 Sep 5;45(35):10654-66. doi: 10.1021/bi060934l. Biochemistry. 2006. PMID: 16939217
-
Positively cooperative binding of zinc ions to Bacillus cereus 569/H/9 beta-lactamase II suggests that the binuclear enzyme is the only relevant form for catalysis.J Mol Biol. 2009 Oct 9;392(5):1278-91. doi: 10.1016/j.jmb.2009.07.092. Epub 2009 Aug 6. J Mol Biol. 2009. PMID: 19665032
-
A variety of roles for versatile zinc in metallo-β-lactamases.Metallomics. 2014 Jul;6(7):1181-97. doi: 10.1039/c4mt00066h. Metallomics. 2014. PMID: 24696003 Review.
-
The reactivity of beta-lactams, the mechanism of catalysis and the inhibition of beta-lactamases.Curr Pharm Des. 1999 Nov;5(11):895-913. Curr Pharm Des. 1999. PMID: 10539995 Review.
Cited by
-
Spectroscopic and mechanistic studies of heterodimetallic forms of metallo-β-lactamase NDM-1.J Am Chem Soc. 2014 May 21;136(20):7273-85. doi: 10.1021/ja410376s. Epub 2014 May 12. J Am Chem Soc. 2014. PMID: 24754678 Free PMC article.
-
Emergence of metal selectivity and promiscuity in metalloenzymes.J Biol Inorg Chem. 2019 Jun;24(4):517-531. doi: 10.1007/s00775-019-01667-0. Epub 2019 May 21. J Biol Inorg Chem. 2019. PMID: 31115763 Review.
-
Role of the Zn1 and Zn2 sites in metallo-beta-lactamase L1.J Am Chem Soc. 2008 Oct 29;130(43):14207-16. doi: 10.1021/ja8035916. Epub 2008 Oct 3. J Am Chem Soc. 2008. PMID: 18831550 Free PMC article.
-
The CphAII protein from Aquifex aeolicus exhibits a metal-dependent phosphodiesterase activity.Extremophiles. 2012 Jan;16(1):45-55. doi: 10.1007/s00792-011-0404-1. Epub 2011 Oct 19. Extremophiles. 2012. PMID: 22009263
-
Structure of New Delhi metallo-β-lactamase 1 (NDM-1).Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Oct 1;67(Pt 10):1160-4. doi: 10.1107/S1744309111029654. Epub 2011 Sep 6. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011. PMID: 22102018 Free PMC article.
References
-
- Frère J. M. β-Lactamases and bacterial resistance to antibiotics. Mol. Microbiol. 1995;16:385–395. - PubMed
-
- Fabiane S. M., Sohi M. K., Wan T., Payne D. J., Bateson J. H., Mitchell T., Sutton B. J. Crystal structure of the zinc-dependent β-lactamase from Bacillus cereus at 1.9 Å resolution: binuclear active site with features of a mononuclear enzyme. Biochemistry. 1998;37:12404–12411. - PubMed
-
- Orellano E. G., Girardini J. E., Cricco J. A., Ceccarelli E. A., Vila A. J. Spectroscopic characterization of a binuclear metal site in Bacillus cereus β-lactamase II. Biochemistry. 1998;37:10173–10180. - PubMed
-
- Paul-Soto R., Bauer R., Frère J. M., Galleni M., Meyer-Klaucke W., Nolting H., Rossolini G. M., de Seny D., Hernandez-Valladares M., Zeppezauer M., Adolph H. W. Mono- and binuclear Zn2+-β-lactamase. Role of the conserved cysteine in the catalytic mechanism. J. Biol. Chem. 1999;274:13242–13249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

