Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat
- PMID: 16962076
- PMCID: PMC1751174
- DOI: 10.1016/j.brainres.2006.08.048
Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat
Abstract
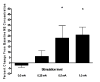
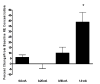
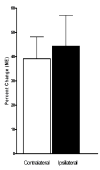
The vagus nerve is an important source of afferent information about visceral states and it provides input to the locus coeruleus (LC), the major source of norepinephrine (NE) in the brain. It has been suggested that the effects of electrical stimulation of the vagus nerve on learning and memory, mood, seizure suppression, and recovery of function following brain damage are mediated, in part, by the release of brain NE. The hypothesis that left vagus nerve stimulation (VNS) at the cervical level results in increased extracellular NE concentrations in the cortex and hippocampus was tested at four stimulus intensities: 0.0, 0.25, 0.5, and 1.0 mA. Stimulation at 0.0 and 0.25 mA had no effect on NE concentrations, while the 0.5 mA stimulation increased NE concentrations significantly in the hippocampus (23%), but not the cortex. However, 1.0 mA stimulation significantly increased NE concentrations in both the cortex (39%) and hippocampus (28%) bilaterally. The increases in NE were transient and confined to the stimulation periods. VNS did not alter NE concentrations in either structure during the inter-stimulation baseline periods. No differences were observed between NE levels in the initial baseline and the post-stimulation baselines. These findings support the hypothesis that VNS increases extracellular NE concentrations in both the hippocampus and cortex.
Figures

Similar articles
-
Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat.J Neurotrauma. 2005 Dec;22(12):1485-502. doi: 10.1089/neu.2005.22.1485. J Neurotrauma. 2005. PMID: 16379585 Free PMC article.
-
Effects of chlordiazepoxide on footshock- and corticotropin-releasing factor-induced increases in cortical and hypothalamic norepinephrine secretion in rats.Neurochem Int. 2008 May;52(6):1220-5. doi: 10.1016/j.neuint.2008.01.002. Epub 2008 Jan 6. Neurochem Int. 2008. PMID: 18280616
-
Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation.J Psychiatry Neurosci. 2009 Jul;34(4):272-80. J Psychiatry Neurosci. 2009. PMID: 19568478 Free PMC article.
-
A literature review on the neurophysiological underpinnings and cognitive effects of transcutaneous vagus nerve stimulation: challenges and future directions.J Neurophysiol. 2020 May 1;123(5):1739-1755. doi: 10.1152/jn.00057.2020. Epub 2020 Mar 25. J Neurophysiol. 2020. PMID: 32208895 Review.
-
Current challenges in reliably targeting the noradrenergic locus coeruleus using transcutaneous auricular vagus nerve stimulation (taVNS).Auton Neurosci. 2021 Dec;236:102900. doi: 10.1016/j.autneu.2021.102900. Epub 2021 Oct 29. Auton Neurosci. 2021. PMID: 34781120 Review.
Cited by
-
Effects of Transcutaneous Auricular Vagus Nerve Stimulation on the P300: Do Stimulation Duration and Stimulation Type Matter?Brain Sci. 2024 Jul 10;14(7):690. doi: 10.3390/brainsci14070690. Brain Sci. 2024. PMID: 39061430 Free PMC article.
-
Corticosterone time-dependently modulates beta-adrenergic effects on long-term potentiation in the hippocampal dentate gyrus.Learn Mem. 2007 May 3;14(5):359-67. doi: 10.1101/lm.527207. Print 2007 May. Learn Mem. 2007. PMID: 17522027 Free PMC article.
-
When the Locus Coeruleus Speaks Up in Sleep: Recent Insights, Emerging Perspectives.Int J Mol Sci. 2022 Apr 30;23(9):5028. doi: 10.3390/ijms23095028. Int J Mol Sci. 2022. PMID: 35563419 Free PMC article. Review.
-
Targeted Vagus Nerve Stimulation for Rehabilitation After Stroke.Front Neurosci. 2019 Mar 29;13:280. doi: 10.3389/fnins.2019.00280. eCollection 2019. Front Neurosci. 2019. PMID: 30983963 Free PMC article. Review.
-
Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders.Front Psychiatry. 2018 Mar 13;9:44. doi: 10.3389/fpsyt.2018.00044. eCollection 2018. Front Psychiatry. 2018. PMID: 29593576 Free PMC article. Review.
References
-
- Amar AP, Levy ML, Mc Comb JG, Apuzzo ML. Vagus nerve stimulation for control of intractable seizures in childhood. Pediatric Neurosurg. 2001;34:218–223. - PubMed
-
- Armitage R, Husain M, Hoffman R, Rush AJ. The effects of vagus nerve stimulation on sleep EEG in depression: a preliminary report. J Psychosom Res. 2003;54:475–482. - PubMed
-
- Ben-Menachem E. Modern management of epilepsy: Vagus nerve stimulation. Baillieres Clin Neurol. 1996;5:841–848. - PubMed
-
- Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002;1:477–482. - PubMed
-
- Bolwig TG. Putative common pathways in therapeutic brain stimulation for affective disorders. CNS Spectr. 2003;8:490–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

