Functional analysis of mutations in TGIF associated with holoprosencephaly
- PMID: 16962354
- PMCID: PMC1820763
- DOI: 10.1016/j.ymgme.2006.07.011
Functional analysis of mutations in TGIF associated with holoprosencephaly
Abstract
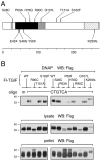
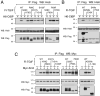
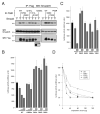
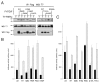
Holoprosencephaly (HPE) is the most common structural malformation of the forebrain and face in humans. Our current understanding of the pathogenesis of HPE attempts to integrate genetic susceptibility, evidenced by mutations in the known HPE genes, with the epigenetic influence of environmental factors. Mutations or deletions of the human TGIF gene have been associated with HPE in multiple population cohorts. Here we examine the functional effects of all previously reported mutations, and describe four additional variants. Of the eleven sequence variations in TGIF, all but four can be demonstrated to be functionally abnormal. In contrast, no potentially pathogenic sequence alterations were detected in the related gene TGIF2. These results provide further evidence of a role for TGIF in HPE and demonstrate the importance of functional analysis of putative disease-associated alleles.
Figures

Similar articles
-
Functions of TGIF homeodomain proteins and their roles in normal brain development and holoprosencephaly.Am J Med Genet C Semin Med Genet. 2018 Jun;178(2):128-139. doi: 10.1002/ajmg.c.31612. Epub 2018 May 11. Am J Med Genet C Semin Med Genet. 2018. PMID: 29749689 Free PMC article.
-
Targeted disruption of Tgif, the mouse ortholog of a human holoprosencephaly gene, does not result in holoprosencephaly in mice.Mol Cell Biol. 2005 May;25(9):3639-47. doi: 10.1128/MCB.25.9.3639-3647.2005. Mol Cell Biol. 2005. PMID: 15831469 Free PMC article.
-
Mutations in TGIF cause holoprosencephaly and link NODAL signalling to human neural axis determination.Nat Genet. 2000 Jun;25(2):205-8. doi: 10.1038/76074. Nat Genet. 2000. PMID: 10835638
-
Mutations in holoprosencephaly.Hum Mutat. 2000;16(2):99-108. doi: 10.1002/1098-1004(200008)16:2<99::AID-HUMU2>3.0.CO;2-0. Hum Mutat. 2000. PMID: 10923031 Review.
-
Holoprosencephaly.Orphanet J Rare Dis. 2007 Feb 2;2:8. doi: 10.1186/1750-1172-2-8. Orphanet J Rare Dis. 2007. PMID: 17274816 Free PMC article. Review.
Cited by
-
Genetics of Combined Pituitary Hormone Deficiency: Roadmap into the Genome Era.Endocr Rev. 2016 Dec;37(6):636-675. doi: 10.1210/er.2016-1101. Epub 2016 Nov 9. Endocr Rev. 2016. PMID: 27828722 Free PMC article. Review.
-
New syndrome of congenital circumferential skin folds associated with multiple congenital anomalies.Pediatr Dermatol. 2012 Jan-Feb;29(1):89-95. doi: 10.1111/j.1525-1470.2011.01403.x. Epub 2011 Oct 13. Pediatr Dermatol. 2012. PMID: 21995818 Free PMC article.
-
Loss of Tgif function causes holoprosencephaly by disrupting the SHH signaling pathway.PLoS Genet. 2012;8(2):e1002524. doi: 10.1371/journal.pgen.1002524. Epub 2012 Feb 23. PLoS Genet. 2012. PMID: 22383895 Free PMC article.
-
TGIF1-Twist1 axis in pancreatic ductal adenocarcinoma.Comput Struct Biotechnol J. 2020 Sep 17;18:2568-2572. doi: 10.1016/j.csbj.2020.09.023. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 33005315 Free PMC article. Review.
-
The molecular genetics of holoprosencephaly.Am J Med Genet C Semin Med Genet. 2010 Feb 15;154C(1):52-61. doi: 10.1002/ajmg.c.30236. Am J Med Genet C Semin Med Genet. 2010. PMID: 20104595 Free PMC article. Review.
References
-
- Muenke M, Beachy PA. Genetics of ventral forebrain development and holoprosencephaly. Cur Opin Genet Dev. 2000;10:262–269. - PubMed
-
- Muenke M, Beachy PA. Holoprosencephaly. In: Scriver CR, Beaudet AL, Valle D, Sly WS, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. Vol. 4. McGraw-Hill; New York, NY: 2001. pp. 6203–6230.
-
- Ming JE, Muenke M. From Homer to hedgehog. Clin Genet. 1998;53:155–163. - PubMed
-
- Cohen M. Perspectives on holoprosencephaly. Part 1. Epidemiology, genetics, and syndromology. Am J Medical Genet. 1989;40:211–235. - PubMed
-
- Brown SA, Warburton D, Brown LY, Yu C-y, Roeder ER, Stengel-Rutkowski S, Hennekam RCM, Muenke M. Holoprosencephaly due to mutations in ZIC2, a homologue of Drosophila odd-paired. Nat Genet. 1998;20:180–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

