Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences
- PMID: 16962364
- PMCID: PMC1820838
- DOI: 10.1016/j.preteyeres.2006.07.003
Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences
Abstract
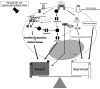
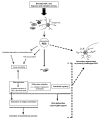
Reactive oxygen species (ROS) are generated as by-products of cellular metabolism, primarily in the mitochondria. Although ROS are essential participants in cell signaling and regulation, when their cellular production overwhelms the intrinsic antioxidant capacity, damage to cellular macromolecules such as DNA, proteins, and lipids ensues. Such a state of "oxidative stress" is thought to contribute to the pathogenesis of a number of neurodegenerative diseases. Growing evidence supports the involvement of oxidative stress as a common component of glaucomatous neurodegeneration in different subcellular compartments of retinal ganglion cells (RGCs). Besides the evidence of direct cytotoxic consequences leading to RGC death, it also seems highly possible that ROS are involved in signaling RGC death by acting as a second messenger and/or modulating protein function by redox modifications of downstream effectors through enzymatic oxidation of specific amino acid residues. Different studies provide cumulating evidence, which supports the association of ROS with different aspects of the neurodegenerative process. Oxidative protein modifications during glaucomatous neurodegeneration increase neuronal susceptibility to damage and also lead to glial dysfunction. Oxidative stress-induced dysfunction of glial cells may contribute to spreading neuronal damage by secondary degeneration. Oxidative stress also promotes the accumulation of advanced glycation end products in glaucomatous tissues. In addition, oxidative stress takes part in the activation of immune response during glaucomatous neurodegeneration, as ROS stimulate the antigen presenting ability of glial cells and also function as co-stimulatory molecules during antigen presentation. By discussing current evidence, this review provides a broad perspective on cellular mechanisms and potential consequences of oxidative stress in glaucoma.
Figures



References
-
- Abu-Amero KK, Morales J, Bosley TM. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2533–2541. - PubMed
-
- Agapova OA, Ricard CS, Salvador-Silva M, Hernandez MR. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human optic nerve head astrocytes. Glia. 2001;33:205–216. - PubMed
-
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10:S18–25. Suppl. - PubMed
-
- Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974;13:771–783. - PubMed
-
- Araie M. Pattern of visual field defects in normal-tension and high-tension glaucoma. Curr Opin Ophthalmol. 1995;6:36–45. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

