Structural transition of the inhibitory region of troponin I within the regulated cardiac thin filament
- PMID: 16962989
- PMCID: PMC1776856
- DOI: 10.1016/j.abb.2006.08.007
Structural transition of the inhibitory region of troponin I within the regulated cardiac thin filament
Abstract
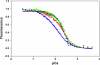
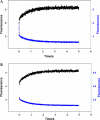
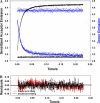
Contraction and relaxation of cardiac muscle are regulated by the inhibitory and regulatory regions of troponin I (cTnI). Our previous FRET studies showed that the inhibitory region of cTnI in isolated troponin experiences a structural transition from a beta-turn/coil motif to an extended conformation upon Ca(2+) activation. During the relaxation process, the kinetics of the reversal of this conformation is coupled to the closing of the Ca(2+)-induced open conformation of the N-domain of troponin C (cTnC) and an interaction between cTnC and cTnI in their interface. We have since extended the structural kinetic study of the inhibitory region to fully regulated thin filament. Single-tryptophan and single-cysteine mutant cTnI(L129W/S151C) was labeled with 1,5-IAEDANS at Cys151, and the tryptophan-AEDANS pair served as a donor-acceptor pair. Labeled cTnI mutant was used to prepare regulated thin filaments. Ca(2+)-induced conformational changes in the segment of Trp129-Cys151 of cTnI were monitored by FRET sensitized acceptor (AEDANS) emission in Ca(2+) titration and stopped-flow measurements. Control experiments suggested energy transfer from endogenous tryptophan residues of actin and myosin S1 to AEDANS attached to Cys151 of cTnI was very small and Ca(2+) independent. The present results show that the rate of Ca(2+)-induced structural transition and Ca(2+) sensitivity of the inhibitory region of cTnI were modified by (1) thin filament formation, (2) the presence of strongly bound S1, and (3) PKA phosphorylation of the N-terminus of cTnI. Ca(2+) sensitivity was not significantly changed by the presence of cTm and actin. However, the cTn-cTm interaction decreased the cooperativity and kinetics of the structural transition within cTnI, while actin filaments elicited opposite effects. The strongly bound S1 significantly increased the Ca(2+) sensitivity and slowed down the kinetics of structural transition. In contrast, PKA phosphorylation of cTnI decreased the Ca(2+) sensitivity and accelerated the structural transition rate of the inhibitory region of cTnI on thin filaments. These results support the idea of a feedback mechanism by strong cross-bridge interaction with actin and provide insights on the molecular basis for the fine tuning of cardiac function by beta-adrenergic stimulation.
Figures



References
-
- Ebashi S, Endo M. Prog. Biophys. Mol. Biol. 1968;18:123–183. - PubMed
-
- Farah CS, Reinach FC. FASEB J. 1995;9:755–767. - PubMed
-
- Liao R, Wang CK, Cheung HC. Biochemistry. 1994;33:12729–12734. - PubMed
-
- Gordon AM, Homsher E, Regnier M. Physiol. Rev. 2000;80:853–924. - PubMed
-
- van Eerd JP, Takahashi K. Biochem. Biophys. Res. Commun. 1975;64:122–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

