Sterol 14alpha-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms
- PMID: 16963187
- PMCID: PMC2324071
- DOI: 10.1016/j.bbagen.2006.07.018
Sterol 14alpha-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms
Abstract
The CYP51 family is an intriguing subject for fundamental P450 structure/function studies and is also an important clinical drug target. This review updates information on the variety of the CYP51 family members, including their physiological roles, natural substrates and substrate preferences, and catalytic properties in vitro. We present experimental support for the notion that specific conserved regions in the P450 sequences represent a CYP51 signature. Two possible roles of CYP51 in P450 evolution are discussed and the major approaches for CYP51 inhibition are summarized.
Figures




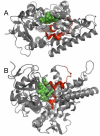

References
-
- Nes WR, McKean MR. Biochemistry of Steroids and Other Iisopentenoids. University Park Press; Baltimore: 1977.
-
- Mitropoulos KA, Gibbons GF, Reeves BE. Lanosterol 14alpha-demethylase. Similarity of the enzyme system from yeast and rat liver. Steroids. 1976;6:821–829. - PubMed
-
- Fiecchi A, Galli-Kienle M, Scala A, Galli G, Grossi Paoletti E, Cattabeni F, Paoletti R. Hydrogen exchange and double bond formation in cholesterol biosynthesis. Proc. R. Soc. Lond. B. Biol. Sci. 1972;180:147–165. - PubMed
-
- Galli-Kienle M, Anastasia M, Cighetti G, Galli G, Fiecchi A. Studies on the 14 alpha-demethylation mechanism in cholesterol biosynthesis. Eur. J. Biochem. 1980;110:93–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

