A specific loop in human DNA polymerase mu allows switching between creative and DNA-instructed synthesis
- PMID: 16963491
- PMCID: PMC1636348
- DOI: 10.1093/nar/gkl457
A specific loop in human DNA polymerase mu allows switching between creative and DNA-instructed synthesis
Abstract
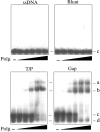
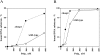
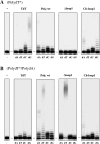
Human DNA polymerase mu (Polmu) is a family X member that has terminal transferase activity but, in spite of a non-orthodox selection of the template information, displays its maximal catalytic efficiency in DNA-templated reactions. As terminal deoxynucleotidyl transferase (TdT), Polmu has a specific loop (loop1) that could provide this enzyme with its terminal transferase activity. When loop1 was deleted, human Polmu lacked TdT activity but improved DNA-binding and DNA template-dependent polymerization. Interestingly, when loop1 from TdT was inserted in Polmu (substituting its cognate loop1), the resulting chimaera displayed TdT activity, preferentially inserting dGTP residues, but had a strongly reduced template-dependent polymerization activity. Therefore, a specialized loop in Polmu, that could adopt alternative conformations, appears to provide this enzyme with a dual capacity: (i) template independency to create new DNA information, in which loop1 would have an active role by acting as a 'pseudotemplate'; (ii) template-dependent polymerization, in which loop1 must allow binding of the template strand. Recent in vivo and in vitro data suggest that such a dual capacity could be advantageous to resolve microhomology-mediated end-joining reactions.
Figures


References
-
- Beard W.A., Wilson S.H. Structural design of a eukaryotic DNA repair polymerase: DNA polymerase beta. Mutat. Res. 2000;460:231–244. - PubMed
-
- Garcia-Diaz M., Bebenek K., Krahn J.M., Blanco L., Kunkel T.A., Pedersen L.C. A structural solution for the DNA polymerase lambda-dependent repair of DNA gaps with minimal homology. Mol. Cell. 2004;13:561–572. - PubMed
-
- Garcia-Diaz M., Bebenek K., Krahn J.M., Kunkel T.A., Pedersen L.C. A closed conformation for the Pol lambda catalytic cycle. Nature Struct. Mol. Biol. 2005;12:97–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

