NOP132 is required for proper nucleolus localization of DEAD-box RNA helicase DDX47
- PMID: 16963496
- PMCID: PMC1636366
- DOI: 10.1093/nar/gkl603
NOP132 is required for proper nucleolus localization of DEAD-box RNA helicase DDX47
Abstract
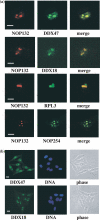
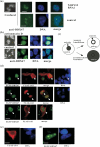
Previously, we described a novel nucleolar protein, NOP132, which interacts with the small GTP binding protein RRAG A. To elucidate the function of NOP132 in the nucleolus, we identified proteins that interact with NOP132 using mass spectrometric methods. NOP132 associated mainly with proteins involved in ribosome biogenesis and RNA metabolism, including the DEAD-box RNA helicase protein, DDX47, whose yeast homolog is Rrp3, which has roles in pre-rRNA processing. Immunoprecipitation of FLAG-tagged DDX47 co-precipitated rRNA precursors, as well as a number of proteins that are probably involved in ribosome biogenesis, implying that DDX47 plays a role in pre-rRNA processing. Introduction of NOP132 small interfering RNAs induced a ring-like localization of DDX47 in the nucleolus, suggesting that NOP132 is required for the appropriate localization of DDX47 within the nucleolus. We propose that NOP132 functions in the recruitment of pre-rRNA processing proteins, including DDX47, to the region where rRNA is transcribed within the nucleolus.
Figures






Similar articles
-
Has1p, a member of the DEAD-box family, is required for 40S ribosomal subunit biogenesis in Saccharomyces cerevisiae.Mol Microbiol. 2004 Apr;52(1):141-58. doi: 10.1111/j.1365-2958.2003.03973.x. Mol Microbiol. 2004. PMID: 15049817
-
Nucleolin functions in the first step of ribosomal RNA processing.EMBO J. 1998 Mar 2;17(5):1476-86. doi: 10.1093/emboj/17.5.1476. EMBO J. 1998. PMID: 9482744 Free PMC article.
-
Interaction of the plant glycine-rich RNA-binding protein MA16 with a novel nucleolar DEAD box RNA helicase protein from Zea mays.Plant J. 2004 Jun;38(6):875-86. doi: 10.1111/j.1365-313X.2004.02095.x. Plant J. 2004. PMID: 15165181
-
Bioinformatic analysis of the nucleolus.Biochem J. 2003 Dec 15;376(Pt 3):553-69. doi: 10.1042/BJ20031169. Biochem J. 2003. PMID: 14531731 Free PMC article. Review.
-
Ribosome biogenesis: of knobs and RNA processing.Exp Cell Res. 2004 May 15;296(1):43-50. doi: 10.1016/j.yexcr.2004.03.016. Exp Cell Res. 2004. PMID: 15120992 Review.
Cited by
-
METTL16 promotes liver cancer stem cell self-renewal via controlling ribosome biogenesis and mRNA translation.J Hematol Oncol. 2024 Feb 1;17(1):7. doi: 10.1186/s13045-024-01526-9. J Hematol Oncol. 2024. PMID: 38302992 Free PMC article.
-
DExD/H-box RNA helicases in ribosome biogenesis.RNA Biol. 2013 Jan;10(1):4-18. doi: 10.4161/rna.21879. Epub 2012 Aug 24. RNA Biol. 2013. PMID: 22922795 Free PMC article. Review.
-
DEAD-ly Affairs: The Roles of DEAD-Box Proteins on HIV-1 Viral RNA Metabolism.Front Cell Dev Biol. 2022 Jun 13;10:917599. doi: 10.3389/fcell.2022.917599. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35769258 Free PMC article. Review.
-
Data augmentation based on the WGAN-GP with data block to enhance the prediction of genes associated with RNA methylation pathways.Sci Rep. 2024 Nov 1;14(1):26321. doi: 10.1038/s41598-024-77107-0. Sci Rep. 2024. PMID: 39487188 Free PMC article.
-
Ribosome assembly factor URB1 contributes to colorectal cancer proliferation through transcriptional activation of ATF4.Cancer Sci. 2021 Jan;112(1):101-116. doi: 10.1111/cas.14643. Epub 2020 Dec 3. Cancer Sci. 2021. PMID: 32888357 Free PMC article.
References
-
- Granneman S., Baserga S.J. Ribosome biogenesis: of knobs and RNA processing. Exp. Cell Res. 2004;296:43–50. - PubMed
-
- Fromont-Racine M., Senger B., Saveanu C., Fasiolo F. Ribosome assembly in eukaryotes. Gene. 2003;313:17–42. - PubMed
-
- Venema J., Tollervey D. Ribosome synthesis in Saccharomyces cerevisiae. Annu. Rev. Genet. 1999;33:261–311. - PubMed
-
- Takahashi N., Yanagida M., Fujiyama S., Hayano T., Isobe T. Proteomic snapshot analyses of preribosomal ribonucleoprotein complexes formed at various stages of ribosome biogenesis in yeast and mammalian cells. Mass Spectrom Rev. 2003;22:287–317. - PubMed
-
- Nishimoto T. Upstream and downstream of ran GTPase. Biol. Chem. 2000;381:397–405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

