Selection of allosteric beta-lactamase mutants featuring an activity regulation by transition metal ions
- PMID: 16963642
- PMCID: PMC2242392
- DOI: 10.1110/ps.062304406
Selection of allosteric beta-lactamase mutants featuring an activity regulation by transition metal ions
Abstract
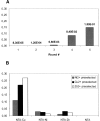
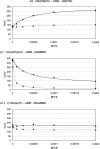
Libraries of phage-displayed beta-lactamase mutants in which up to three loops have been engineered by genetic introduction of random peptide sequences or by randomization of the wild-type sequence have been submitted to selection protocols designed to find mutants in which binding of transition metal ions to the engineered secondary binding site leads to significant effects on the enzymatic activity. A double-selection protocol was applied: The phage-displayed libraries were first selected for transition metal ions affinity by panning on IMAC support, then a second selection step was applied to isolate mutants that have retained significant catalytic activity. The analysis of the kinetic properties of mutants in the presence of nickel, copper, or zinc ions allowed isolation of a few mutants whose activity was either enhanced or inhibited by factors up to three and >10, respectively, in a metal-specific manner. A remarkable mutant exhibiting differential allosteric regulation depending on the metal was found. Its activity was activated by nickel ion binding, inhibited by cupric ion binding, and nearly unaffected by zinc ions. These observations point to an interesting potential for up- or down-regulation of activity within a monomeric enzyme by binding to an "allosteric site" relatively remote from the active site.
Figures
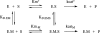
Similar articles
-
Active TEM-1 beta-lactamase mutants with random peptides inserted in three contiguous surface loops.Protein Sci. 2006 Oct;15(10):2323-34. doi: 10.1110/ps.062303606. Epub 2006 Sep 8. Protein Sci. 2006. PMID: 16963643 Free PMC article.
-
Engineering allosteric regulation into the hinge region of a circularly permuted TEM-1 beta-lactamase.Protein Eng Des Sel. 2010 Sep;23(9):699-709. doi: 10.1093/protein/gzq041. Epub 2010 Jun 30. Protein Eng Des Sel. 2010. PMID: 20591901
-
Selection of beta-lactamases and penicillin binding mutants from a library of phage displayed TEM-1 beta-lactamase randomly mutated in the active site omega-loop.J Mol Biol. 2000 Jan 21;295(3):527-40. doi: 10.1006/jmbi.1999.3376. J Mol Biol. 2000. PMID: 10623544
-
Selection of metalloenzymes by catalytic activity using phage display and catalytic elution.Chembiochem. 2001 Apr 2;2(4):253-9. doi: 10.1002/1439-7633(20010401)2:4<253::AID-CBIC253>3.0.CO;2-6. Chembiochem. 2001. PMID: 11828452
-
Metalloallostery and Transition Metal Signaling: Bioinorganic Copper Chemistry Beyond Active Sites.Angew Chem Int Ed Engl. 2023 Mar 6;62(11):e202213644. doi: 10.1002/anie.202213644. Epub 2023 Jan 18. Angew Chem Int Ed Engl. 2023. PMID: 36653724 Free PMC article. Review.
Cited by
-
Phage display: selecting straws instead of a needle from a haystack.Molecules. 2011 Jan 19;16(1):790-817. doi: 10.3390/molecules16010790. Molecules. 2011. PMID: 21248664 Free PMC article. Review.
-
Ligand binding and allostery can emerge simultaneously.Protein Sci. 2007 May;16(5):929-37. doi: 10.1110/ps.062706007. Epub 2007 Mar 30. Protein Sci. 2007. PMID: 17400921 Free PMC article.
-
Crystallization and preliminary X-ray diffraction analysis of kanamycin-binding β-lactamase in complex with its ligand.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Jun 1;67(Pt 6):703-6. doi: 10.1107/S1744309111013832. Epub 2011 May 26. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011. PMID: 21636917 Free PMC article.
-
Active TEM-1 beta-lactamase mutants with random peptides inserted in three contiguous surface loops.Protein Sci. 2006 Oct;15(10):2323-34. doi: 10.1110/ps.062303606. Epub 2006 Sep 8. Protein Sci. 2006. PMID: 16963643 Free PMC article.
-
On the origin of non-specific binders isolated in the selection of phage display peptide libraries.Front Microbiol. 2025 Jun 4;16:1571679. doi: 10.3389/fmicb.2025.1571679. eCollection 2025. Front Microbiol. 2025. PMID: 40535010 Free PMC article. Review.
References
-
- Benito, A., Feliu, J.X., Villaverde, A. 1996. β-Galactosidase enzymatic activity as a molecular probe to detect specific antibodies. J. Biol. Chem. 271: 21251–21256. - PubMed
-
- Binz, H.K., Amstutz, P., Pluckthun, A. 2005. Engineering novel binding proteins from nonimmunoglobulin domains. Nat. Biotechnol. 23: 1257–1268. - PubMed
-
- Brennan, C., Christianson, K., Surowi, T., Mandecki, W. 1994. Modulation of enzyme activity by antibody binding to an alkaline phosphatase-epitope hybrid protein. Protein Eng. 7: 509–514. - PubMed
-
- Browner, M.F., Hackos, D., Fletterick, R. 1994. Identification of the molecular trigger for allosteric activation in glycogen phosphorylase. Nat. Struct. Biol. 1: 327–333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

