Selection of allosteric beta-lactamase mutants featuring an activity regulation by transition metal ions
- PMID: 16963642
- PMCID: PMC2242392
- DOI: 10.1110/ps.062304406
Selection of allosteric beta-lactamase mutants featuring an activity regulation by transition metal ions
Abstract
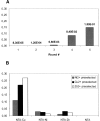
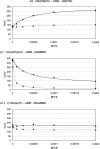
Libraries of phage-displayed beta-lactamase mutants in which up to three loops have been engineered by genetic introduction of random peptide sequences or by randomization of the wild-type sequence have been submitted to selection protocols designed to find mutants in which binding of transition metal ions to the engineered secondary binding site leads to significant effects on the enzymatic activity. A double-selection protocol was applied: The phage-displayed libraries were first selected for transition metal ions affinity by panning on IMAC support, then a second selection step was applied to isolate mutants that have retained significant catalytic activity. The analysis of the kinetic properties of mutants in the presence of nickel, copper, or zinc ions allowed isolation of a few mutants whose activity was either enhanced or inhibited by factors up to three and >10, respectively, in a metal-specific manner. A remarkable mutant exhibiting differential allosteric regulation depending on the metal was found. Its activity was activated by nickel ion binding, inhibited by cupric ion binding, and nearly unaffected by zinc ions. These observations point to an interesting potential for up- or down-regulation of activity within a monomeric enzyme by binding to an "allosteric site" relatively remote from the active site.
Figures
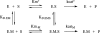
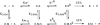
References
-
- Benito, A., Feliu, J.X., Villaverde, A. 1996. β-Galactosidase enzymatic activity as a molecular probe to detect specific antibodies. J. Biol. Chem. 271: 21251–21256. - PubMed
-
- Binz, H.K., Amstutz, P., Pluckthun, A. 2005. Engineering novel binding proteins from nonimmunoglobulin domains. Nat. Biotechnol. 23: 1257–1268. - PubMed
-
- Brennan, C., Christianson, K., Surowi, T., Mandecki, W. 1994. Modulation of enzyme activity by antibody binding to an alkaline phosphatase-epitope hybrid protein. Protein Eng. 7: 509–514. - PubMed
-
- Browner, M.F., Hackos, D., Fletterick, R. 1994. Identification of the molecular trigger for allosteric activation in glycogen phosphorylase. Nat. Struct. Biol. 1: 327–333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

