CXCR2 ligands and G-CSF mediate PKCalpha-induced intraepidermal inflammation
- PMID: 16964312
- PMCID: PMC1560349
- DOI: 10.1172/JCI27514
CXCR2 ligands and G-CSF mediate PKCalpha-induced intraepidermal inflammation
Abstract
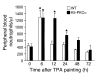
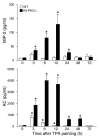
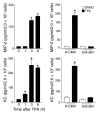
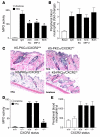
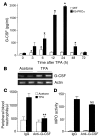
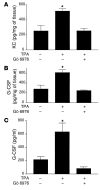
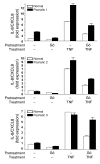
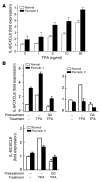
Transgenic mice overexpressing PKCalpha in the epidermis (K5-PKCalpha mice) exhibit an inducible severe intraepidermal neutrophilic inflammation and systemic neutrophilia when PKCalpha is activated by topical 12-O-tetradecanoylphorbol-13-acetate (TPA). This inducible model of cutaneous inflammation was used to define mediators of skin inflammation that may have clinical relevance. Activation of cutaneous PKCalpha increased the production of the chemotactic factors cytokine-induced neutrophil chemoattractant (KC) and macrophage inflammatory protein 2 (MIP-2) in murine plasma. TPA treatment of cultured K5-PKCalpha keratinocytes also released KC and MIP-2 into culture supernatants through an NF-kappaB-dependent pathway. MIP-2 and KC mediated the infiltration of neutrophils into the epidermis, since this was prevented by ablating CXCR2 in K5-PKCalpha mice or administering neutralizing antibodies against KC or MIP-2. The neutrophilia resulted from PKCalpha-mediated upregulation of cutaneous G-CSF released into the plasma independent of CXCR2. These responses could be inhibited by topical treatment with a PKCalpha-selective inhibitor. Inhibiting PKCalpha also reduced the basal and TNF-alpha- or TPA-induced expression of CXCL8 in cultured psoriatic keratinocytes, suggesting that PKCalpha activity may contribute to psoriatic inflammation. Thus, skin can be the source of circulating factors that have both local and systemic consequences, and these factors, their receptors, and possibly PKCalpha could be therapeutic targets for inhibition of cutaneous inflammation.
Figures
References
-
- Murphy P.M., et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 2000;52:145–176. - PubMed
-
- Murphy P.M. International Union of Pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacol. Rev. 2002;54:227–229. - PubMed
-
- Zlotnik A., Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. - PubMed
-
- Bozic C.R., et al. Expression and biologic characterization of the murine chemokine KC. . J. Immunol. 1995;154:6048–6057. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

