The other pigment cell: specification and development of the pigmented epithelium of the vertebrate eye
- PMID: 16965267
- PMCID: PMC1564434
- DOI: 10.1111/j.1600-0749.2006.00318.x
The other pigment cell: specification and development of the pigmented epithelium of the vertebrate eye
Abstract
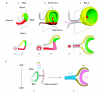
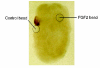
Vertebrate retinal pigment epithelium (RPE) cells are derived from the multipotent optic neuroepithelium, develop in close proximity to the retina, and are indispensible for eye organogenesis and vision. Recent advances in our understanding of RPE development provide evidence for how critical signaling factors operating in dorso-ventral and distal-proximal gradients interact with key transcription factors to specify three distinct domains in the budding optic neuroepithelium: the distal future retina, the proximal future optic stalk/optic nerve, and the dorsal future RPE. Concomitantly with domain specification, the eye primordium progresses from a vesicle to a cup, RPE pigmentation extends towards the ventral side, and the future ciliary body and iris form from the margin zone between RPE and retina. While much has been learned about the molecular networks controlling RPE cell specification, key questions concerning the cell proliferative parameters in RPE and the subsequent morphogenetic events still need to be addressed in greater detail.
Figures

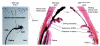
References
-
- Ahmad I, Tang L, Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem. Biophys. Res. Commun. 2000;270:517–521. - PubMed
-
- Arnheiter H. Evolutionary biology. Eyes viewed from the skin. Nature. 1998;391:632–633. - PubMed
-
- Arnheiter H, Hou L, Nguyen MT, Nakayama A, Champagne B, Hallsson JH, Bismuth K. The role of microphthalmia in pigment cell development. In: Ortonne J.p., Ballotti R., editors. Mechanisms of Suntanning. Martin Dunitz Publication; London: 2002. pp. 49–63.
-
- Arnheiter H, Hou L, Nguyen MTT, Bismuth K, Csermely T, Murakami H, Skuntz S, Liu W, Bharti K. Mitf—A matter of life and death for the developing melanocyte. In: Hearing V, Leong SPL, editors. From Melanocytes to Malignant Melanoma. Humana Press; Totowa, NJ: 2006. pp. 27–49.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

