Hepatic progenitor cells, stem cells, and AFP expression in models of liver injury
- PMID: 16965562
- PMCID: PMC2517380
- DOI: 10.1111/j.1365-2613.2006.00485.x
Hepatic progenitor cells, stem cells, and AFP expression in models of liver injury
Abstract
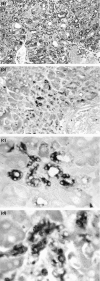
Adult hepatocytes and liver-cell progenitors play a role in restoring liver tissue after injury. For the study of progenitor cells in liver repair, experimental models included (a) surgical removal of liver tissue by partial hepatectomy; (b) acute injury by carbontetrachloride; (c) acute injury by d-galactosamine (GalN) and N-nitrosomorpholine (NNM); and (d) chemical hepatocarcinogenesis by feeding NNM in low and high doses. Serological and immunohistological detection of alpha-fetoprotein gene expression served to follow pathways of cellular differentiation. Stem cells were not required in models of surgical removal of parenchyma and in carbon tetrachloride intoxication of adult hepatocytes. In contrast, regeneration of liver occurred through biliary epithelial cells in injuries induced by GalN and NNM. These biliary epithelial cells, collectively called oval cells, are most probably derived from the canals of Hering. Proliferating bile duct cells reached a level of differentiation with reactivation of foetal genes and significant alpha-1-fetoprotein (AFP) synthesis signalling a certain degree of retrodifferentiation with potential stemness. Due to the same embryonic origin of bile ducts and hepatocytes, biliary epithelium and its proliferating progeny (oval cells) have a defined role in liver regeneration as a transit and amplification compartment. In their early proliferation stage, oval cells were heavily engaged in DNA synthesis ([3H]thymidine labelling). Pulse-chase experiments during experimental hepatocarcinogenesis exhibited their development into hepatocytes with high risk for transformation and leading to foci of altered hepatocytes. Hepatocellular carcinomas may arise either from proliferating/differentiating oval cells or from adult hepatocytes; both cell types have stem-like properties. AFP-positive and AFP-negative carcinomas occurred in the same liver. They may represent random clonal origin. The heterogeneity of phenotypic marker (AFP) correlated with a process of retrodifferentiation.
Figures




Similar articles
-
Heterogeneity of alpha-fetoprotein(AFP) and albumin containing cells in normal and pathological permissive states for AFP production: AFP containing cells induced in adult rats recapitulate the appearance of AFP containing hepatocytes in fetal rats.Oncodev Biol Med. 1980;1(2):93-105. Oncodev Biol Med. 1980. PMID: 6169057
-
Activation, proliferation, and differentiation of progenitor cells into hepatocytes in the D-galactosamine model of liver regeneration.Am J Pathol. 1993 Dec;143(6):1606-20. Am J Pathol. 1993. PMID: 7504886 Free PMC article.
-
Modulation of keratin 14 and alpha-fetoprotein expression during hepatic oval cell proliferation and liver regeneration.J Cell Physiol. 1994 Jun;159(3):475-84. doi: 10.1002/jcp.1041590312. J Cell Physiol. 1994. PMID: 7514611
-
EpCAM and the biology of hepatic stem/progenitor cells.Am J Physiol Gastrointest Liver Physiol. 2015 Feb 15;308(4):G233-50. doi: 10.1152/ajpgi.00069.2014. Epub 2014 Dec 4. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25477371 Free PMC article. Review.
-
Development and molecular composition of the hepatic progenitor cell niche.Dan Med J. 2013 May;60(5):B4640. Dan Med J. 2013. PMID: 23673270 Review.
Cited by
-
Ameliorating effect of encapsulated hepatocyte-like cells derived from umbilical cord in high mannuronic alginate scaffolds on acute liver failure in rats.Iran J Basic Med Sci. 2018 Sep;21(9):928-935. doi: 10.22038/IJBMS.2018.27928.6847. Iran J Basic Med Sci. 2018. PMID: 30524693 Free PMC article.
-
Primary Hepatic Neuroendocrine Tumor Mimicking Ruptured Hepatocellular Carcinoma with AFP Elevation: A Case Report and Literature Review.Onco Targets Ther. 2020 Feb 3;13:975-979. doi: 10.2147/OTT.S236728. eCollection 2020. Onco Targets Ther. 2020. PMID: 32099400 Free PMC article.
-
Founder cells for hepatocytes during liver regeneration: from identification to application.Cell Mol Life Sci. 2020 Aug;77(15):2887-2898. doi: 10.1007/s00018-020-03457-3. Epub 2020 Feb 14. Cell Mol Life Sci. 2020. PMID: 32060582 Free PMC article. Review.
-
SGCAST: symmetric graph convolutional auto-encoder for scalable and accurate study of spatial transcriptomics.Brief Bioinform. 2023 Nov 22;25(1):bbad490. doi: 10.1093/bib/bbad490. Brief Bioinform. 2023. PMID: 38171928 Free PMC article.
-
Liver regeneration as treatment target for severe alcoholic hepatitis.World J Gastroenterol. 2022 Aug 28;28(32):4557-4573. doi: 10.3748/wjg.v28.i32.4557. World J Gastroenterol. 2022. PMID: 36157937 Free PMC article. Review.
References
-
- Alison MR. Regulation of hepatic growth. Physiol. Rev. 1986;66:499–541. - PubMed
-
- Alison M, Golding M, Lalani EN, Nagy P, Thorgeirsson S, Sarraf C. Wholesale hepatocytic differentiation in the rat from ductular oval cells, the progeny of biliary stem cells. J. Hepatol. 1997;26:343–352. - PubMed
-
- Alison MR, Poulsom R, Jeffery R, et al. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. - PubMed
-
- Anderson DJ, Gage FH, Weissman IL. Can stem cells cross lineage boundaries? Nat. Med. 2001;7:393–395. - PubMed
-
- Anilkumar TV, Golding M, Edwards RJ, Lalani EN, Sarraf CE, Alison MR. The resistant hepatocyte model of carcinogenesis in the rat: the apparent independent development of oval cell proliferation and early nodules. Carcinogenesis. 1995;16:845–853. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

