High expression of insulin-like growth factor binding protein-3 is correlated with lower portal invasion and better prognosis in human hepatocellular carcinoma
- PMID: 16965600
- PMCID: PMC11158442
- DOI: 10.1111/j.1349-7006.2006.00322.x
High expression of insulin-like growth factor binding protein-3 is correlated with lower portal invasion and better prognosis in human hepatocellular carcinoma
Abstract
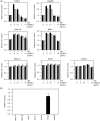
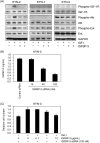
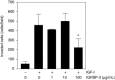
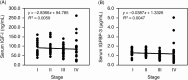
Insulin-like growth factor binding protein-3 (IGFBP-3) modulates cell proliferation of various cancer cell types. However, it remains unclear how IGF-IGFBP-3-signaling is involved in growth and progression of hepatocellular carcinoma (HCC). The aim of the present study was to evaluate the role of IGFBP-3 in HCC. Type 1 receptor for IGF (IGF-1R) was expressed at various levels in the seven lines examined, but IGF-2R was not expressed. Of the seven lines, the growth of HAK-1B, KIM-1, KYN-2 and HepG2 cells was stimulated in a dose-dependent manner by the exogenous addition of IGF-I or IGF-II, but the HAK-1A, KYN-1 and KYN-3 cell lines showed no growth. Exogenous addition of IGFBP-3 markedly blocked IGF-I and IGF-II-stimulated cell growth of KYN-2 and HepG2 cells, and moderately stimulated that of KIM-1 and HAK-1B cells, but no growth of the KYN-1, KYN-3 and HAK-1A cell lines was observed. IGF-I enhanced the phosphorylation of IGF-1R, Akt and Erk1/2 in KYN-2 cells, and coadministration of IGFBP-3 blocked all types of activation by IGF-I investigated here. In contrast, no such activation by IGF-I was detected in KYN-3 cells. IGFBP-3 also suppressed IGF-I-induced cell invasion by KYN-2 cells. Moreover, we were able to observe the apparent expression of IGFBP-3 in KYN-3 cells, but not in the other six cell lines. Furthermore reduced expression of IGFBP-3, but not that of IGF-1R, was significantly correlated with tumor size, histological differentiation, capsular invasion and portal venous invasion. Low expression of IGFBP-3 was independently associated with poor survival. IGFBP-3 could be a molecular target of intrinsic importance for further development of novel therapeutic strategy against HCC.
Figures


References
-
- Shimada M, Takenaka K, Gion T et al. Prognosis of recurrent hepatocellular carcinoma: a 10‐year surgical experience in Japan. Gastroenterology 1996; 111: 720–6. - PubMed
-
- Adachi E, Maehara S, Tsujita E et al. Clinicopathologic risk factors for recurrence after a curative hepatic resection for hepatocellular carcinoma. Surgery 2002; 131: 148–52. - PubMed
-
- Scharf JG, Schmidt‐Sandte W, Pahernik SA, Ramadori G, Braulke T, Hartmann H. Characterization of the insulin‐like growth factor axis in a human hepatoma cell line (PLC). Carcinogenesis 1998; 19: 2121–8. - PubMed
-
- LeRoith D, Werner H, Beitner‐Johnson D, Roberts CT Jr. Molecular and cellular aspects of the insulin‐like growth factor I receptor. Endocr Rev 1995; 16: 143–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

