Pharmacogenetic impact of polymorphisms in the coding region of the UGT1A1 gene on SN-38 glucuronidation in Japanese patients with cancer
- PMID: 16965601
- PMCID: PMC11158602
- DOI: 10.1111/j.1349-7006.2006.00321.x
Pharmacogenetic impact of polymorphisms in the coding region of the UGT1A1 gene on SN-38 glucuronidation in Japanese patients with cancer
Abstract
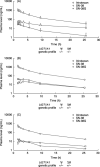
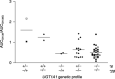
Pharmacogenetic testing for UDP-glucuronosyltransferase (UGT) 1A1*28, a promoter variant of the UGT1A1 gene, is now carried out clinically to estimate the risk of irinotecan-associated toxicity. We studied the clinical significance of UGT1A1*6 and UGT1A1*27, two variants in exon 1 of the UGT1A1 gene that are found mainly in Asians. The study group comprised 46 Japanese patients who received various regimens of chemotherapy including irinotecan at doses from 50 to 180 mg/m(2). Pharmacogenetic relationships were explored between the UGT1A1 genotype and the ratio of the area under the plasma concentration-time curve (AUC) of the active metabolite of irinotecan (SN-38) to that of SN-38 glucuronide (SN-38G), used as a surrogate for UGT1A1 activity (AUC(SN-38)/AUC(SN-38G)). No patient was homozygous for UGT1A1*28, and none had UGT1A1*27. Two were heterozygous for UGT1A1*28. Two were homozygous and 15 heterozygous for UGT1A1*6, all of whom were wild type with respect to UGT1A1*28. Two patients were simultaneously heterozygous for UGT1A1*28 and UGT1A1*6, present on different chromosomes. The other 25 patients had none of the variants studied. The two patients simultaneously heterozygous for UGT1A1*28 and UGT1A1*6 and the two patients homozygous for UGT1A1*6 had significantly higher AUC(SN-38)/AUC(SN-38G) ratios than the others (P = 0.0039). Concurrence of UGT1A1*28 and UGT1A1*6, even when heterozygous, altered the disposition of irinotecan remarkably, potentially increasing susceptibility to toxicity. Patients homozygous for UGT1A1*6 should also be carefully monitored. UGT1A1 polymorphisms in the coding region of the UGT1A1 gene should be genotyped in addition to testing for UGT1A1*28 to more accurately predict irinotecan-related toxicity, at least in Asian patients.
Figures
References
-
- Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med 2005; 352: 476–87. - PubMed
-
- Noda K, Nishiwaki Y, Kawahara M et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small‐cell lung cancer. N Engl J Med 2002; 346: 85–91. - PubMed
-
- Tadokoro J, Hasegawa H, Hayakawa K. Post‐marketing surveillance (PMS) of all patients treated with irinotecan in Japan: clinical experience and ADR profile of 13 935 patients. Proc Am Soc Clin Oncol 2002; 21: 259. - PubMed
-
- Kubota K, Nishiwaki Y, Ohashi Y. The Four‐Arm Cooperative Study (FACS) for advanced non‐small‐cell lung cancer (NSCLC). J Clin Oncol 2004; 22: 618.
-
- Mathijssen RH, Van Alphen RJ, Verweij J et al. Clinical pharmacokinetics and metabolism of irinotecan (CPT‐11). Clin Cancer Res 2001; 7: 2182–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

