Ascaris suum-derived products suppress mucosal allergic inflammation in an interleukin-10-independent manner via interference with dendritic cell function
- PMID: 16966410
- PMCID: PMC1698059
- DOI: 10.1128/IAI.00720-06
Ascaris suum-derived products suppress mucosal allergic inflammation in an interleukin-10-independent manner via interference with dendritic cell function
Abstract
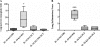
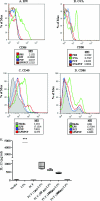
We have previously demonstrated that protection from allergic inflammation by Ascaris suum infection was characterized by a global increase in interleukin-10 (IL-10) and the development of protective CD4(+)/CD25(+) T cells (L. Schopf, S. Luccioli, V. Bundoc, P. Justice, C. C. Chan, B. J. Wetzel, H. H. Norris, J. F. Urban, Jr., and A. Keane-Myers, Investig. Ophthalmol. Vis. Sci. 46:2772-2780, 2005). Here, we used A. suum pseudocoelomic fluid (PCF) in lieu of infection to define molecular mechanisms of allergic protection in a mouse model of allergic inflammation. Mice were sensitized with ragweed (RW) and PCF (RW/PCF), PCF alone, or RW alone and then challenged intratracheally, intranasally, and supraocularly with RW. Histological examination of the eyes and lungs, analysis of the bronchoalveolar lavage fluid (BALF), and characterization of ex vivo cytokine responses were performed to determine allergic inflammatory responses. RW/PCF-treated mice had suppressed allergic immune responses compared to mice given RW alone. To investigate whether IL-10 was involved in PCF-mediated allergic protection, similar experiments were performed using mice genetically deficient for IL-10. Persistent protection from allergic disease was observed in the absence of IL-10, indicating the primary mechanism of PCF protection is IL-10 independent. Ex vivo and in vitro analysis of PCF-treated dendritic cells (DC) demonstrated reduced activation receptor expression and cytokine production in response to either RW or lipopolysaccharide stimulation. These findings extend previous studies that showed infection with A. suum alters expression of allergic disease and suggest that PCF can contribute to this effect by interference with DC function.
Figures

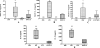


Similar articles
-
Differential modulation of allergic eye disease by chronic and acute ascaris infection.Invest Ophthalmol Vis Sci. 2005 Aug;46(8):2772-80. doi: 10.1167/iovs.04-0899. Invest Ophthalmol Vis Sci. 2005. PMID: 16043850
-
Inhibitory receptor gp49B regulates eosinophil infiltration during allergic inflammation.J Leukoc Biol. 2007 Dec;82(6):1531-41. doi: 10.1189/jlb.1106667. Epub 2007 Aug 30. J Leukoc Biol. 2007. PMID: 17761953
-
Endogenous interleukin-10 produced by antigen-irrelevant cells promotes the development of experimental murine allergic conjunctivitis.Int Arch Allergy Immunol. 2007;144(1):79-84. doi: 10.1159/000102618. Epub 2007 May 15. Int Arch Allergy Immunol. 2007. PMID: 17505142
-
Mucosal tolerance and immunity: regulating the development of allergic disease and asthma.Int Arch Allergy Immunol. 2003 Feb;130(2):108-18. doi: 10.1159/000069012. Int Arch Allergy Immunol. 2003. PMID: 12673064 Review.
-
IL-10: a potential therapy for allergic inflammation?Immunol Today. 1997 Jun;18(6):277-80. doi: 10.1016/s0167-5699(97)80023-0. Immunol Today. 1997. PMID: 9190113 Review. No abstract available.
Cited by
-
The yin and yang of human soil-transmitted helminth infections.Int J Parasitol. 2021 Dec;51(13-14):1243-1253. doi: 10.1016/j.ijpara.2021.11.001. Epub 2021 Nov 10. Int J Parasitol. 2021. PMID: 34774540 Free PMC article. Review.
-
In vivo and in vitro studies using Clonorchis sinensis adult-derived total protein (CsTP) on cellular function and inflammatory effect in mouse and cell model.Parasitol Res. 2020 May;119(5):1641-1652. doi: 10.1007/s00436-020-06651-1. Epub 2020 Apr 14. Parasitol Res. 2020. PMID: 32285266
-
Changes of cytokine mRNA expression and IgG responses in rats infected with Capillaria hepatica.Korean J Parasitol. 2007 Jun;45(2):95-102. doi: 10.3347/kjp.2007.45.2.95. Korean J Parasitol. 2007. PMID: 17570971 Free PMC article.
-
Vernal keratoconjunctivitis in school children in Rwanda and its association with socio-economic status: a population-based survey.Am J Trop Med Hyg. 2011 Oct;85(4):711-7. doi: 10.4269/ajtmh.2011.11-0291. Am J Trop Med Hyg. 2011. PMID: 21976577 Free PMC article.
-
Parasites and asthma.Parasitol Res. 2017 Sep;116(9):2373-2383. doi: 10.1007/s00436-017-5548-1. Epub 2017 Jul 8. Parasitol Res. 2017. PMID: 28689246 Review.
References
-
- Aksoy, E., C. S. Zouain, F. Vanhoutte, J. Fontaine, N. Pavelka, N. Thieblemont, F. Willems, P. Ricciardi-Castagnoli, M. Goldman, M. Capron, B. Ryffel, and F. Trottein. 2005. Double-stranded RNAs from the helminth parasite Schistosoma activate TLR3 in dendritic cells. J. Biol. Chem. 280:277-283. - PubMed
-
- Araujo, M. I., B. S. Hoppe, M. Medeiros, Jr., and E. M. Carvalho. 2004. Schistosoma mansoni infection modulates the immune response against allergic and auto-immune diseases. Mem. Inst. Oswaldo Cruz 99:27-32. - PubMed
-
- Araujo, M. I., A. A. Lopes, M. Medeiros, A. A. Cruz, L. Sousa-Atta, D. Sole, and E. M. Carvalho. 2000. Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int. Arch. Allergy Immunol. 123:145-148. - PubMed
-
- Arruda, L. K., and A. B. Santos. 2005. Immunologic responses to common antigens in helminthic infections and allergic disease. Curr. Opin. Allergy Clin. Immunol. 5:399-402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

