The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions
- PMID: 16966423
- PMCID: PMC2064336
- DOI: 10.1083/jcb.200604015
The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions
Abstract
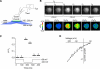
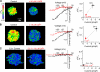
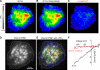
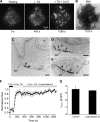
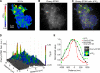
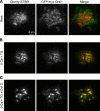
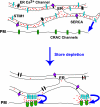
The activation of store-operated Ca(2+) entry by Ca(2+) store depletion has long been hypothesized to occur via local interactions of the endoplasmic reticulum (ER) and plasma membrane, but the structure involved has never been identified. Store depletion causes the ER Ca(2+) sensor stromal interacting molecule 1 (STIM1) to form puncta by accumulating in junctional ER located 10-25 nm from the plasma membrane (see Wu et al. on p. 803 of this issue). We have combined total internal reflection fluorescence (TIRF) microscopy and patch-clamp recording to localize STIM1 and sites of Ca(2+) influx through open Ca(2+) release-activated Ca(2+) (CRAC) channels in Jurkat T cells after store depletion. CRAC channels open only in the immediate vicinity of STIM1 puncta, restricting Ca(2+) entry to discrete sites comprising a small fraction of the cell surface. Orai1, an essential component of the CRAC channel, colocalizes with STIM1 after store depletion, providing a physical basis for the local activation of Ca(2+) influx. These studies reveal for the first time that STIM1 and Orai1 move in a coordinated fashion to form closely apposed clusters in the ER and plasma membranes, thereby creating the elementary unit of store-operated Ca(2+) entry.
Figures


Similar articles
-
Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane.J Cell Biol. 2006 Sep 11;174(6):803-13. doi: 10.1083/jcb.200604014. J Cell Biol. 2006. PMID: 16966422 Free PMC article.
-
Nanoscale patterning of STIM1 and Orai1 during store-operated Ca2+ entry.Proc Natl Acad Sci U S A. 2015 Oct 6;112(40):E5533-42. doi: 10.1073/pnas.1515606112. Epub 2015 Sep 8. Proc Natl Acad Sci U S A. 2015. PMID: 26351694 Free PMC article.
-
Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation.Nature. 2008 Jul 24;454(7203):538-42. doi: 10.1038/nature07065. Epub 2008 Jul 2. Nature. 2008. PMID: 18596693 Free PMC article.
-
Remodelling of the endoplasmic reticulum during store-operated calcium entry.Biol Cell. 2011 Aug;103(8):365-80. doi: 10.1042/BC20100152. Biol Cell. 2011. PMID: 21736554 Review.
-
Some assembly required: constructing the elementary units of store-operated Ca2+ entry.Cell Calcium. 2007 Aug;42(2):163-72. doi: 10.1016/j.ceca.2007.03.003. Epub 2007 May 11. Cell Calcium. 2007. PMID: 17499354 Free PMC article. Review.
Cited by
-
Emerging roles of store-operated Ca²⁺ entry through STIM and ORAI proteins in immunity, hemostasis and cancer.Channels (Austin). 2013 Sep-Oct;7(5):379-91. doi: 10.4161/chan.24302. Epub 2013 Mar 19. Channels (Austin). 2013. PMID: 23511024 Free PMC article. Review.
-
STIM proteins: dynamic calcium signal transducers.Nat Rev Mol Cell Biol. 2012 Sep;13(9):549-65. doi: 10.1038/nrm3414. Nat Rev Mol Cell Biol. 2012. PMID: 22914293 Free PMC article. Review.
-
Regulatory mechanisms controlling store-operated calcium entry.Front Physiol. 2023 Dec 19;14:1330259. doi: 10.3389/fphys.2023.1330259. eCollection 2023. Front Physiol. 2023. PMID: 38169682 Free PMC article. Review.
-
STIM1 enhances SR Ca2+ content through binding phospholamban in rat ventricular myocytes.Proc Natl Acad Sci U S A. 2015 Aug 25;112(34):E4792-801. doi: 10.1073/pnas.1423295112. Epub 2015 Aug 10. Proc Natl Acad Sci U S A. 2015. PMID: 26261328 Free PMC article.
-
BIN2 orchestrates platelet calcium signaling in thrombosis and thrombo-inflammation.J Clin Invest. 2020 Nov 2;130(11):6064-6079. doi: 10.1172/JCI136457. J Clin Invest. 2020. PMID: 32750041 Free PMC article.
References
-
- Al-Mohanna, F.A., E.J. Pettit, and M.B. Hallett. 1997. Does actin polymerization status modulate Ca2+ storage in human neutrophils? Release and coalescence of Ca2+ stores by cytochalasins. Exp. Cell Res. 234:379–387. - PubMed
-
- Cheng, H., W.J. Lederer, and M.B. Cannell. 1993. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 262:740–744. - PubMed
-
- Fagan, K.A., R. Mahey, and D.M. Cooper. 1996. Functional co-localization of transfected Ca2+-stimulable adenylyl cyclases with capacitative Ca2+ entry sites. J. Biol. Chem. 271:12438–12444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

