PKA-activated ApAF-ApC/EBP heterodimer is a key downstream effector of ApCREB and is necessary and sufficient for the consolidation of long-term facilitation
- PMID: 16966424
- PMCID: PMC2064337
- DOI: 10.1083/jcb.200512066
PKA-activated ApAF-ApC/EBP heterodimer is a key downstream effector of ApCREB and is necessary and sufficient for the consolidation of long-term facilitation
Abstract
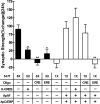
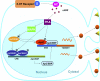
Long-term memory requires transcriptional regulation by a combination of positive and negative transcription factors. Aplysia activating factor (ApAF) is known to be a positive transcription factor that forms heterodimers with ApC/EBP and ApCREB2. How these heterodimers are regulated and how they participate in the consolidation of long-term facilitation (LTF) has not, however, been characterized. We found that the functional activation of ApAF required phosphorylation of ApAF by PKA on Ser-266. In addition, ApAF lowered the threshold of LTF by forming a heterodimer with ApCREB2. Moreover, once activated by PKA, the ApAF-ApC/EBP heterodimer transactivates enhancer response element-containing genes and can induce LTF in the absence of CRE- and CREB-mediated gene expression. Collectively, these results suggest that PKA-activated ApAF-ApC/EBP heterodimer is a core downstream effector of ApCREB in the consolidation of LTF.
Figures
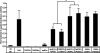




References
-
- Abel, T., and E. Kandel. 1998. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res. Brain Res. Rev. 26:360–378. - PubMed
-
- Alberini, C.M., M. Ghirardi, R. Metz, and E.R. Kandel. 1994. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 76:1099–1114. - PubMed
-
- Barondes, S.H., and H.D. Cohen. 1968. Memory impairment after subcutaneous injection of acetoxycycloheximide. Science. 160:556–557. - PubMed
-
- Bartsch, D., M. Ghirardi, P.A. Skehel, K.A. Karl, S.P. Herder, M. Chen, C.H. Bailey, and E.R. Kandel. 1995. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 83:979–992. - PubMed
-
- Bartsch, D., A. Casadio, K.A. Karl, P. Serodio, and E.R. Kandel. 1998. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 95:211–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

