State-dependent block of BK channels by synthesized shaker ball peptides
- PMID: 16966472
- PMCID: PMC2151574
- DOI: 10.1085/jgp.200609521
State-dependent block of BK channels by synthesized shaker ball peptides
Abstract
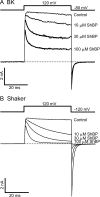
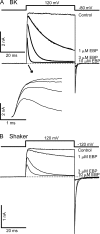
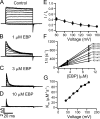
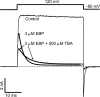
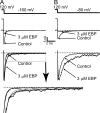
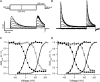
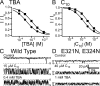
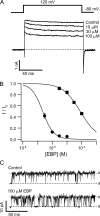
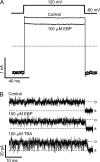
Crystal structures of potassium channels have strongly corroborated an earlier hypothetical picture based on functional studies, in which the channel gate was located on the cytoplasmic side of the pore. However, accessibility studies on several types of ligand-sensitive K(+) channels have suggested that their activation gates may be located near or within the selectivity filter instead. It remains to be determined to what extent the physical location of the gate is conserved across the large K(+) channel family. Direct evidence about the location of the gate in large conductance calcium-activated K(+) (BK) channels, which are gated by both voltage and ligand (calcium), has been scarce. Our earlier kinetic measurements of the block of BK channels by internal quaternary ammonium ions have raised the possibility that they may lack a cytoplasmic gate. We show in this study that a synthesized Shaker ball peptide (ShBP) homologue acts as a state-dependent blocker for BK channels when applied internally, suggesting a widening at the intracellular end of the channel pore upon gating. This is consistent with a gating-related conformational change at the cytoplasmic end of the pore-lining helices, as suggested by previous functional and structural studies on other K(+) channels. Furthermore, our results from two BK channel mutations demonstrate that similar types of interactions between ball peptides and channels are shared by BK and other K(+) channel types.
Figures



References
-
- Bers, D.M. 1982. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. Am. J. Physiol. 242:C404–C408. - PubMed

