The two ATP binding sites of cystic fibrosis transmembrane conductance regulator (CFTR) play distinct roles in gating kinetics and energetics
- PMID: 16966475
- PMCID: PMC2151577
- DOI: 10.1085/jgp.200609622
The two ATP binding sites of cystic fibrosis transmembrane conductance regulator (CFTR) play distinct roles in gating kinetics and energetics
Abstract
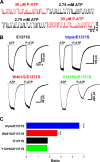
Cystic fibrosis transmembrane conductance regulator (CFTR), a member of the ABC (ATP binding cassette) transporter family, is a chloride channel whose activity is controlled by protein kinase-dependent phosphorylation. Opening and closing (gating) of the phosphorylated CFTR is coupled to ATP binding and hydrolysis at CFTR's two nucleotide binding domains (NBD1 and NBD2). Recent studies present evidence that the open channel conformation reflects a head-to-tail dimerization of CFTR's two NBDs as seen in the NBDs of other ABC transporters (Vergani et al., 2005). Whether these two ATP binding sites play an equivalent role in the dynamics of NBD dimerization, and thus in gating CFTR channels, remains unsettled. Based on the crystal structures of NBDs, sequence alignment, and homology modeling, we have identified two critical aromatic amino acids (W401 in NBD1 and Y1219 in NBD2) that coordinate the adenine ring of the bound ATP. Conversion of the W401 residue to glycine (W401G) has little effect on the sensitivity of the opening rate to [ATP], but the same mutation at the Y1219 residue dramatically lowers the apparent affinity for ATP by >50-fold, suggesting distinct roles of these two ATP binding sites in channel opening. The W401G mutation, however, shortens the open time constant. Energetic analysis of our data suggests that the free energy of ATP binding at NBD1, but not at NBD2, contributes significantly to the energetics of the open state. This kinetic and energetic asymmetry of CFTR's two NBDs suggests an asymmetric motion of the NBDs during channel gating. Opening of the channel is initiated by ATP binding at the NBD2 site, whereas separation of the NBD dimer at the NBD1 site constitutes the rate-limiting step in channel closing.
Figures


Similar articles
-
Stable ATP binding mediated by a partial NBD dimer of the CFTR chloride channel.J Gen Physiol. 2010 May;135(5):399-414. doi: 10.1085/jgp.201010399. J Gen Physiol. 2010. PMID: 20421370 Free PMC article.
-
Prolonged nonhydrolytic interaction of nucleotide with CFTR's NH2-terminal nucleotide binding domain and its role in channel gating.J Gen Physiol. 2003 Sep;122(3):333-48. doi: 10.1085/jgp.200308798. J Gen Physiol. 2003. PMID: 12939393 Free PMC article.
-
On the mechanism of gating defects caused by the R117H mutation in cystic fibrosis transmembrane conductance regulator.J Physiol. 2016 Jun 15;594(12):3227-44. doi: 10.1113/JP271723. Epub 2016 Mar 23. J Physiol. 2016. PMID: 26846474 Free PMC article.
-
Gating of the CFTR Cl- channel by ATP-driven nucleotide-binding domain dimerisation.J Physiol. 2009 May 15;587(Pt 10):2151-61. doi: 10.1113/jphysiol.2009.171595. Epub 2009 Mar 30. J Physiol. 2009. PMID: 19332488 Free PMC article. Review.
-
ATP hydrolysis cycles and the gating of CFTR Cl- channels.Acta Physiol Scand Suppl. 1998 Aug;643:247-56. Acta Physiol Scand Suppl. 1998. PMID: 9789567 Review.
Cited by
-
Recovery of ΔF508-CFTR Function by Citrate.Nutrients. 2022 Oct 14;14(20):4283. doi: 10.3390/nu14204283. Nutrients. 2022. PMID: 36296967 Free PMC article.
-
The CFTR ion channel: gating, regulation, and anion permeation.Cold Spring Harb Perspect Med. 2013 Jan 1;3(1):a009498. doi: 10.1101/cshperspect.a009498. Cold Spring Harb Perspect Med. 2013. PMID: 23284076 Free PMC article.
-
Allosteric coupling between the intracellular coupling helix 4 and regulatory sites of the first nucleotide-binding domain of CFTR.PLoS One. 2013 Sep 18;8(9):e74347. doi: 10.1371/journal.pone.0074347. eCollection 2013. PLoS One. 2013. PMID: 24058550 Free PMC article.
-
Degenerate ABC composite site is stably glued together by trapped ATP.J Gen Physiol. 2010 May;135(5):395-8. doi: 10.1085/jgp.201010443. J Gen Physiol. 2010. PMID: 20421369 Free PMC article. No abstract available.
-
Estimating the true stability of the prehydrolytic outward-facing state in an ABC protein.Elife. 2023 Oct 2;12:e90736. doi: 10.7554/eLife.90736. Elife. 2023. PMID: 37782012 Free PMC article.
References
-
- Aleksandrov, L., A. Mengos, X.-B. Chang, A. Aleksandrov, and J.R. Riordan. 2001. Differential interactions of nucleotides at the two nucleotide binding domains of CFTR. J. Biol. Chem. 276:12918–12923. - PubMed
-
- Aleksandrov, L., A.A. Aleksandrov, X.-B. Chang, and J.R. Riordan. 2002. The first nucleotide binding domain of cystic fibrosis transmembrane conductance regulator is a site of stable nucleotide interaction, whereas the second is a site of rapid turnover. J. Biol. Chem. 277:15419–15425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

