Combination of Ca2+ -activated K+ channel blockers inhibits acetylcholine-evoked nitric oxide release in rat superior mesenteric artery
- PMID: 16967048
- PMCID: PMC2014669
- DOI: 10.1038/sj.bjp.0706886
Combination of Ca2+ -activated K+ channel blockers inhibits acetylcholine-evoked nitric oxide release in rat superior mesenteric artery
Abstract
Background and purpose: The present study investigated whether calcium-activated K+ channels are involved in acetylcholine-evoked nitric oxide (NO) release and relaxation.
Experimental approach: Simultaneous measurements of NO concentration and relaxation were performed in rat superior mesenteric artery and endothelial cell membrane potential and intracellular calcium ([Ca2+]i) were measured.
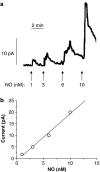
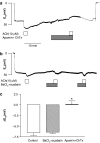
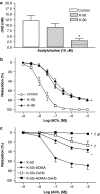
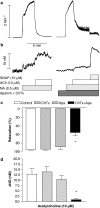
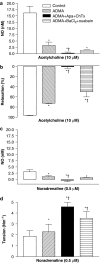
Key results: A combination of apamin plus charybotoxin, which are, respectively, blockers of small-conductance and of intermediate- and large-conductance Ca2+ -activated K channels abolished acetylcholine (10 microM)-evoked hyperpolarization of endothelial cell membrane potential. Acetylcholine-evoked NO release was reduced by 68% in high K+ (80 mM) and by 85% in the presence of apamin plus charybdotoxin. In noradrenaline-contracted arteries, asymmetric dimethylarginine (ADMA), an inhibitor of NO synthase inhibited acetylcholine-evoked NO release and relaxation. However, only further addition of oxyhaemoglobin or apamin plus charybdotoxin eliminated the residual acetylcholine-evoked NO release and relaxation. Removal of extracellular calcium or an inhibitor of calcium influx channels, SKF96365, abolished acetylcholine-evoked increase in NO concentration and [Ca2+]i. Cyclopiazonic acid (CPA, 30 microM), an inhibitor of sarcoplasmic Ca2+ -ATPase, caused a sustained NO release in the presence, but only a transient increase in the absence, of extracellular calcium. Incubation with apamin and charybdotoxin did not change acetylcholine or CPA-induced increases in [Ca2+]i, but inhibited the sustained NO release induced by CPA.
Conclusions and implications: Acetylcholine increases endothelial cell [Ca2+]i by release of stored calcium and calcium influx resulting in activation of apamin and charybdotoxin-sensitive K channels, hyperpolarization and release of NO in the rat superior mesenteric artery.
Figures



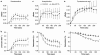
Similar articles
-
Opening of small and intermediate calcium-activated potassium channels induces relaxation mainly mediated by nitric-oxide release in large arteries and endothelium-derived hyperpolarizing factor in small arteries from rat.J Pharmacol Exp Ther. 2011 Dec;339(3):842-50. doi: 10.1124/jpet.111.179242. Epub 2011 Aug 31. J Pharmacol Exp Ther. 2011. PMID: 21880870
-
Cellular target of voltage and calcium-dependent K(+) channel blockers involved in EDHF-mediated responses in rat superior mesenteric artery.Br J Pharmacol. 2001 Nov;134(5):1021-8. doi: 10.1038/sj.bjp.0704348. Br J Pharmacol. 2001. PMID: 11682450 Free PMC article.
-
Different modulation by Ca2+-activated K+ channel blockers and herbimycin of acetylcholine- and flow-evoked vasodilatation in rat mesenteric small arteries.Br J Pharmacol. 2003 Apr;138(8):1562-70. doi: 10.1038/sj.bjp.0705214. Br J Pharmacol. 2003. PMID: 12721112 Free PMC article.
-
A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery.Br J Pharmacol. 1997 Dec;122(8):1573-84. doi: 10.1038/sj.bjp.0701546. Br J Pharmacol. 1997. PMID: 9422801 Free PMC article.
-
Interactions between endothelium-derived relaxing factors in the rat hepatic artery: focus on regulation of EDHF.Br J Pharmacol. 1998 Jul;124(5):992-1000. doi: 10.1038/sj.bjp.0701893. Br J Pharmacol. 1998. PMID: 9692786 Free PMC article.
Cited by
-
Openers of small conductance calcium-activated potassium channels selectively enhance NO-mediated bradykinin vasodilatation in porcine retinal arterioles.Br J Pharmacol. 2010 Jul;160(6):1496-508. doi: 10.1111/j.1476-5381.2010.00803.x. Br J Pharmacol. 2010. PMID: 20590639 Free PMC article.
-
Extracellular l-arginine Enhances Relaxations Induced by Opening of Calcium-Activated SKCa Channels in Porcine Retinal Arteriole.Int J Mol Sci. 2019 Apr 25;20(8):2032. doi: 10.3390/ijms20082032. Int J Mol Sci. 2019. PMID: 31027156 Free PMC article.
-
Role of in vivo vascular redox in resistance arteries.Hypertension. 2015 Jan;65(1):130-9. doi: 10.1161/HYPERTENSIONAHA.114.04473. Epub 2014 Oct 13. Hypertension. 2015. PMID: 25312439 Free PMC article.
-
Endothelial TRPV4/Cx43 Signaling Complex Regulates Vasomotor Tone in Resistance Arteries.bioRxiv [Preprint]. 2024 Jul 25:2024.07.25.604930. doi: 10.1101/2024.07.25.604930. bioRxiv. 2024. Update in: J Physiol. 2025 Aug;603(15):4345-4366. doi: 10.1113/JP285194. PMID: 39091840 Free PMC article. Updated. Preprint.
-
Subtype-Selective Positive Modulation of KCa2.3 Channels Increases Cilia Length.ACS Chem Biol. 2022 Aug 19;17(8):2344-2354. doi: 10.1021/acschembio.2c00469. Epub 2022 Aug 10. ACS Chem Biol. 2022. PMID: 35947779 Free PMC article.
References
-
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. - PubMed
-
- Bolz SS, Fisslthaler B, Pieperhoff S, De Wit C, Fleming I, Busse R, et al. Antisense oligonucleotides against cytochrome P450 2C8 attenuate EDHF-mediated Ca(2+) changes and dilation in isolated resistance arteries. FASEB J. 2000;14:255–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

