Tissue inhibitor of metalloproteinase-2 (TIMP-2) regulates neuromuscular junction development via a beta1 integrin-mediated mechanism
- PMID: 16967503
- PMCID: PMC2982212
- DOI: 10.1002/neu.20315
Tissue inhibitor of metalloproteinase-2 (TIMP-2) regulates neuromuscular junction development via a beta1 integrin-mediated mechanism
Abstract
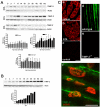
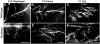
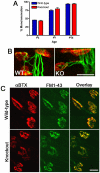
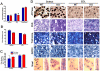
Extracellular matrix (ECM) molecules play critical roles in muscle function by participating in neuromuscular junction (NMJ) development and the establishment of stable, cytoskeleton-associated adhesions required for muscle contraction. Matrix metalloproteinases (MMPs) are neutral endopeptidases that degrade all ECM components. While the role of MMPs and their inhibitors, the tissue inhibitor of metalloproteinases (TIMPs), has been investigated in many tissues, little is known about their role in muscle development and mature function. TIMP-2 -/- mice display signs of muscle weakness. Here, we report that TIMP-2 is expressed at the NMJ and its expression is greater in fast-twitch (extensor digitorum longus, EDL) than slow-twitch (soleus) muscle. EDL muscle mass is reduced in TIMP-2-/- mice without a concomitant change in fiber diameter or number. The TIMP-2-/- phenotype is not likely due to increased ECM proteolysis because net MMP activity is actually reduced in TIMP-2-/- muscle. Most strikingly, TIMP-2 colocalizes with beta1 integrin at costameres in the wild-type EDL and beta1 integrin expression is significantly reduced in TIMP-2-/- EDL. We propose that reduced beta1 integrin in fast-twitch muscle may be associated with destabilized ECM-cytoskeletal interactions required for muscle contraction in TIMP-2-/- muscle; thus, explaining the muscle weakness. Given that fast-twitch fibers are lost in muscular dystrophies and age-related sarcopenia, if TIMP-2 regulates mechanotransduction in an MMP-independent manner it opens new potential therapeutic avenues.
(c) 2006 Wiley Periodicals, Inc.
Figures

References
-
- Bewick GS, Reid B, Jawaid S, Hatcher T, Shanley L. Postnatal emergence of mature release properties in terminals of rat fast- and slow-twitch muscles. Eur. J. Neurosci. 2004;19:2967–2976. - PubMed
-
- Boppart MD, Burkin DJ, Kaufman SJ. α7β1 Integrin Regulates Mechanotransduction and Prevents Skeletal Muscle Injury. Am. J. Physiol. Cell Physiol. 2006 E-pub. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. - PubMed
-
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

