De novo malignancies following liver transplantation: a case-control study with long-term follow-up
- PMID: 16968488
- PMCID: PMC4050657
- DOI: 10.1111/j.1399-0012.2006.00527.x
De novo malignancies following liver transplantation: a case-control study with long-term follow-up
Abstract
Background: Long-term survival data on de novo malignancy are limited following orthotopic liver transplantation (OLT) when compared with controls without malignancies.
Methods: Over a 12 yr period at our institution, 50 of 1043 patients (4.8%) who underwent OLT were identified to have 53 de novo malignancies. The clinical characteristics and survival of these patients were retrospectively reviewed and compared with a control cohort of 50 OLT recipients without malignancy matched with the incidence cases by age, year of OLT, sex, and type of liver disease.
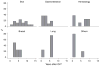
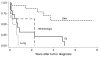
Results: Chronic hepatitis C, alcohol and primary sclerosing cholangitis were the three leading causes of liver disease. Skin cancer was the most common malignancy (32%), followed by gastrointestinal (21%), including five small bowel tumors, and hematologic malignancies (17%). The cases and controls were not significantly different in the immunosuppressive regimen (p = 0.42) or the number of rejection episodes (p = 0.92). The five- and 10-year Kaplan-Meier survival rates for the cases were 77% and 34%, respectively, vs. 84% and 70%, respectively, for the controls (p = 0.02 by log-rank test). Patients with skin cancers had survival similar to the controls, but significantly better than non-skin cancers (p = 0.0001). The prognosis for patients with gastrointestinal tumors was poor, with a median survival of 8.5 months after the diagnosis.
Conclusion: In this single institutional study, de novo malignancies after OLT were uncommon. Patients with non-skin cancer after OLT had diminished long-term survival compared with the controls. Our results differ from other reports in the high incidence of gastrointestinal malignancies with attendant poor prognosis.
Figures


Similar articles
-
Long term follow-up and outcome of liver transplantation for alcoholic liver disease: a single center case-control study.J Clin Gastroenterol. 2010 Jan;44(1):52-7. doi: 10.1097/MCG.0b013e3181a390a8. J Clin Gastroenterol. 2010. PMID: 19581813
-
Low recurrence of preexisting extrahepatic malignancies after liver transplantation.Liver Transpl. 2008 Jun;14(6):789-98. doi: 10.1002/lt.21434. Liver Transpl. 2008. PMID: 18412260
-
Risk Factors and Outcomes of De Novo Cancers (Excluding Nonmelanoma Skin Cancer) After Liver Transplantation for Primary Sclerosing Cholangitis.Transplantation. 2017 Aug;101(8):1859-1866. doi: 10.1097/TP.0000000000001725. Transplantation. 2017. PMID: 28272287 Free PMC article.
-
De novo malignancies after liver transplantation: a major cause of late death.Liver Transpl. 2001 Nov;7(11 Suppl 1):S109-18. doi: 10.1053/jlts.2001.28645. Liver Transpl. 2001. PMID: 11689783 Review.
-
Incidence, risk factors and outcome of de novo tumors in liver transplant recipients focusing on alcoholic cirrhosis.World J Hepatol. 2015 May 8;7(7):942-53. doi: 10.4254/wjh.v7.i7.942. World J Hepatol. 2015. PMID: 25954477 Free PMC article. Review.
Cited by
-
De novo malignancies after liver transplantation: The effect of immunosuppression-personal data and review of literature.World J Gastroenterol. 2019 Sep 21;25(35):5356-5375. doi: 10.3748/wjg.v25.i35.5356. World J Gastroenterol. 2019. PMID: 31558879 Free PMC article.
-
Post-transplant malignancies in alcoholic liver disease.Transl Gastroenterol Hepatol. 2020 Apr 5;5:30. doi: 10.21037/tgh.2019.11.18. eCollection 2020. Transl Gastroenterol Hepatol. 2020. PMID: 32258534 Free PMC article. Review.
-
Betel quid chewing leads to the development of unique de novo malignancies in liver transplant recipients, a retrospective single center study in Taiwan.Medicine (Baltimore). 2016 Sep;95(37):e4901. doi: 10.1097/MD.0000000000004901. Medicine (Baltimore). 2016. PMID: 27631265 Free PMC article.
-
Neoplastic disease after liver transplantation: Focus on de novo neoplasms.World J Gastroenterol. 2015 Aug 7;21(29):8753-68. doi: 10.3748/wjg.v21.i29.8753. World J Gastroenterol. 2015. PMID: 26269665 Free PMC article. Review.
-
Extrahepatic Malignancies and Liver Transplantation: Current Status.J Clin Exp Hepatol. 2021 Jul-Aug;11(4):494-500. doi: 10.1016/j.jceh.2020.10.008. Epub 2020 Oct 24. J Clin Exp Hepatol. 2021. PMID: 34276155 Free PMC article. Review.
References
-
- Yao FYK, Bass NM. Liver transplantation. In: Yamada T, Alpers DH, Kaplowitz N, Laine L, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 4. Philadelphia: Lippincott Williams & Wilkins; 2003. p. 2468.
-
- Sheil AGR. Malignancy following liver transplantation: a report from the Australian combined liver transplant registry. Transplant Proc. 1995;27:1247. - PubMed
-
- Penn I. Posttransplantation de novo tumors in liver allograft recipients. Liver Transpl Surg. 1996;2:52. - PubMed
-
- Jonas S, Rayes N, Neumann U, et al. De novo malignancies after liver transplantation using tacrolimus-based protocols or cyclosporine-based quadruple immunosuppression with an interleukin-2 receptor antibody or anti-thymocyte globulin. Cancer. 1997;80:1141. - PubMed
-
- Kelly DM, Emre S, Guy SR, Miller CM, Schwartz MR, Sheiner PA. Liver transplant recipients are not at increased risk for non-lymphoid solid organ tumors. Cancer. 1998;83:1237. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

