Hedgehog modulates cell cycle regulators in stem cells to control hematopoietic regeneration
- PMID: 16968775
- PMCID: PMC1599924
- DOI: 10.1073/pnas.0604568103
Hedgehog modulates cell cycle regulators in stem cells to control hematopoietic regeneration
Abstract
The signals that control the regenerative ability of hematopoietic stem cells (HSCs) in response to damage are unknown. Here, we demonstrate that downstream activation of the Hedgehog (Hh) signaling pathway induces cycling and expansion of primitive bone marrow hematopoietic cells under homeostatic conditions and during acute regeneration. However, this effect is at the expense of HSC function, because continued Hh activation during regeneration represses expression of specific cell cycle regulators, leading to HSC exhaustion. In vivo treatment with an inhibitor of the Hh pathway rescues these transcriptional and functional defects in HSCs. Our study establishes Hh signaling as a regulator of the HSC cell cycle machinery that balances hematopoietic homeostasis and regeneration in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
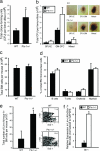

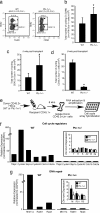

References
-
- Hodgson GS, Bradley TR, Radley JM. Exp Hematol. 1982;10:26–35. - PubMed
-
- Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Science. 2000;287:1804–1808. - PubMed
-
- Roy S, Ingham PW. J Cell Sci. 2002;115:4393–4397. - PubMed
-
- Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH. Development (Cambridge, UK) 2001;128:1717–1730. - PubMed
-
- Gering M, Patient R. Dev Cell. 2005;8:389–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

