IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin
- PMID: 16968902
- PMCID: PMC1785078
- DOI: 10.1182/blood-2006-02-002873
IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin
Abstract
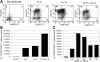
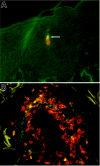
Regulatory T cells (Tregs) are crucial for the induction and maintenance of self-tolerance and are present in peripheral tissues such as skin and gut under normal, noninflamed conditions. We report isolation and expansion of the Treg population resident in normal human skin. Cutaneous Tregs expressed high levels of CD25, L-selectin, GITR, FOXP3, and intracellular CTLA-4, low levels of CD69, and high levels of the skin-homing addressins CLA, CCR4, and CCR6. Skin Tregs suppressed the proliferation of CD25(lo) T cells from the same skin sample in response to CD3 and CD28 antibodies. Suppression was dependent on cell contact and not affected by neutralizing antibodies to interleukin-10 (IL-10) and transforming growth factor-beta (TGF-beta). Surprisingly, cutaneous Tregs proliferated in an antigen-independent manner when cultured in contact with dermal fibroblasts and IL-15, conditions similar to those found in chronically inflamed skin. We hypothesize that local proliferation of Tregs may occur within inflamed skin and could serve as a brake for cutaneous inflammation as well as a mechanism for the homeostatic proliferation of natural Tregs that has been observed within intact organisms.
Conflict of interest statement
Conflict-of-interest statement: The authors declare they have no conflicts of interest.
Figures


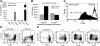

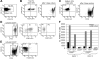
References
-
- Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–755. - PubMed
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. - PubMed
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
-
- Powell BR, Buist NR, Stenzel P. An X-linked syndrome of diarrhea, polyendocrinopathy, and fatal infection in infancy. J Pediatr. 1982;100:731–737. - PubMed
-
- Satake N, Nakanishi M, Okano M, et al. A Japanese family of X-linked auto-immune enteropathy with haemolytic anaemia and polyendocrinopathy. Eur J Pediatr. 1993;152:313–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

