International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy
- PMID: 16968944
- PMCID: PMC3471216
- DOI: 10.1124/pr.58.3.3
International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy
Abstract
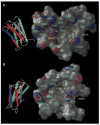
There have been many advances in our knowledge about different aspects of P2Y receptor signaling since the last review published by our International Union of Pharmacology subcommittee. More receptor subtypes have been cloned and characterized and most orphan receptors de-orphanized, so that it is now possible to provide a basis for a future subdivision of P2Y receptor subtypes. More is known about the functional elements of the P2Y receptor molecules and the signaling pathways involved, including interactions with ion channels. There have been substantial developments in the design of selective agonists and antagonists to some of the P2Y receptor subtypes. There are new findings about the mechanisms underlying nucleotide release and ectoenzymatic nucleotide breakdown. Interactions between P2Y receptors and receptors to other signaling molecules have been explored as well as P2Y-mediated control of gene transcription. The distribution and roles of P2Y receptor subtypes in many different cell types are better understood and P2Y receptor-related compounds are being explored for therapeutic purposes. These and other advances are discussed in the present review.
Figures




References
-
- Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, et al. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–55. - PMC - PubMed
-
- Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64:445–475. - PubMed
-
- Abbracchio MP, Verderio C. Pathophysiological roles of P2 receptors in glial cells. Novartis Found Symp. 2006;276:91–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

