Safety and tolerability of elubaquine (bulaquine, CDRI 80/53) for treatment of Plasmidium vivax malaria in Thailand
- PMID: 16969059
- PMCID: PMC2532664
- DOI: 10.3347/kjp.2006.44.3.221
Safety and tolerability of elubaquine (bulaquine, CDRI 80/53) for treatment of Plasmidium vivax malaria in Thailand
Abstract
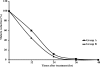
We conducted a study to compare the safety and tolerability of anti-relapse drugs elubaquine and primaquine against Plasmodium vivax malaria. After standard therapy with chloroquine, 30 mg/kg given over 3 days, 141 patients with P. vivax infection were randomized to receive primaquine or elubaquine. The 2 treatment regimens were primaquine 30 mg once daily for 7 days (group A, n = 71), and elubaquine 25 mg once daily for 7 days (group B, n = 70). All patients cleared parasitemia within 7 days after chloroquine treatment. Among patients treated with primaquine, one patient relapsed on day 26; no relapse occurred with elubaquine treatement. Both drugs were well tolerated. Adverse effects occurred only in patients with G6PD deficiency who were treated with primaquine (group A, n = 4), whose mean hematocrit fell significantly on days 7, 8 and 9 (P = 0.015, 0.027, and 0.048, respectively). No significant change in hematocrit was observed in patients with G6PD deficiency who were treated with elubaquine (group B, n = 3) or in patients with normal G6PD. In conclusion, elubaquine, as anti-relapse therapy for P. vivax malaria, was as safe and well tolerated as primaquine and did not cause clinically significant hemolysis.
Figures
References
-
- Anklesaria PS, Ashar VJ, Kshirsagar NA, Gupta KC. Comparison of the effect of compound CDRI 80/53 [N1-(3 acetyl-4-5-dihydro-2 furanyl)-N4-(6-methoxy-8-quinolinyl)1,4-pentanediamine] with primaquine on human erythrocytes in vitro. In: Kumar S, Sen AK, Dutta GP, Sharma RN, editors. Tropical Diseases: Molecular Biology and Control Strategies. New Delhi, India: Publication and Information Directorate, Council of Scientific and Industrial Research; 1994.
-
- Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–1345. - PubMed
-
- Baird JK, Leksana B, Masbar S, Fryauff DJ, Sutanihardja MA, Suradi, Wignall FS, Hoffman SL. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am J Trop Med Hyg. 1997;56:621–626. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous