Fate of embryonic stem cells transplanted into the deafened mammalian cochlea
- PMID: 16970279
- PMCID: PMC1810231
- DOI: 10.3727/000000006783981819
Fate of embryonic stem cells transplanted into the deafened mammalian cochlea
Abstract
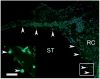
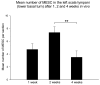
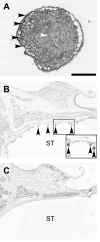
Spiral ganglion neurons (SGNs), the primary afferent neurons of the cochlea, degenerate following a sensorineural hearing loss (SNHL) due to lack of trophic support normally received from hair cells. Cell transplantation is emerging as a potential strategy for inner ear rehabilitation, as injected cells may be able to replace damaged SGNs in the deafened cochlea. An increase in the number of surviving SGNs may result in improved efficacy of cochlear implants (CIs). We examined the survival of partially differentiated mouse embryonic stem cells (MESCs), following xenograft transplantation into the deafened guinea pig cochlea (n=15). Cells were delivered directly into the left scala tympani via microinjection through the round window. Small numbers of MESCs were detected in the scala tympani for up to 4 weeks following transplantation and a proportion of these cells retained expression of neurofilament protein 68 kDa in vivo. While this delivery method requires refinement for effective long-term replacement of damaged SGNs, small numbers of MESCs were capable of survival in the deafened mammalian cochlea for up to 4 weeks, without causing an inflammatory tissue response.
Figures






References
-
- Andrew JK. Department of Otolaryngology. Melbourne: University of Melbourne; 2003. Rehabilitation of the Deafened Auditory Nerve With Schwann Cell Transplantation; pp. 1–114.
-
- Coleman B, Hardman J, Crook J, de Silva M, Epp S, Coco A, Shepherd RK. Frontiers in Otorhinolaryngology. Noosa; 2004. Survival and migration of partially differentiated stem cells in the adult guinea pig cochlea.
-
- Coleman B, Hardman J, de Silva M, Crook J, Shepherd RK. International Society for Stem Cell Research. San Francisco; 2005. Strategies for stem cell-based therapy in the mammalian cochlea.
-
- Duckert LG, Duvall AJ., 3rd Cochlear communication routes in the guinea pig-spiral ganglia and osseous spiral laminae: an electron microscope study using microsphere tracers. Otolaryngology. 1978;86:ORL434–446. - PubMed
-
- Ernfors P, Duan ML, ElShamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nature Medicine. 1996;2:463–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

