TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists
- PMID: 16971522
- PMCID: PMC6674601
- DOI: 10.1523/JNEUROSCI.1246-06.2006
TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists
Abstract
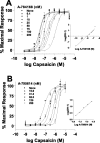
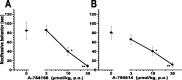
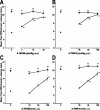
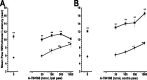
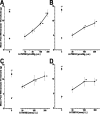
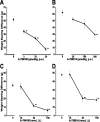
Vanilloid receptor type 1 (TRPV1) is a ligand-gated nonselective cation channel that is considered to be an important integrator of various pain stimuli such as endogenous lipids, capsaicin, heat, and low pH. In addition to expression in primary afferents, TRPV1 is also expressed in the CNS. To test the hypothesis that the CNS plays a differential role in the effect of TRPV1 antagonists in various types of pain, the analgesic effects of two TRPV1 antagonists with similar in vitro potency but different CNS penetration were compared in vivo. Oral administration of either A-784168 (1-[3-(trifluoromethyl)pyridin-2-yl]-N-[4-(trifluoromethylsulfonyl)phenyl]-1,2,3,6-tetrahydropyridine-4-carboxamide) (good CNS penetration) or A-795614 (N-1H-indazol-4-yl-N'-[(1R)-5-piperidin-1-yl-2,3-dihydro-1H-inden-1-yl]urea) (poor CNS penetration) blocked capsaicin-induced acute pain with the same potency. In complete Freund's adjuvant (CFA)-induced chronic inflammatory pain, oral administration of either compound blocked thermal hyperalgesia with similar potency. Furthermore, intraplantar or intrathecal administration of A-784168 blocked CFA-induced thermal hyperalgesia, suggesting that both peripheral and CNS TRPV1 receptors may play a role in inflammatory thermal hyperalgesia. The effects of the two TRPV1 antagonists were further assessed in models presumably mediated by central sensitization, including CFA- and capsaicin-induced mechanical allodynia and osteoarthritic pain. In these models, the potency of the two compounds was similar after intrathecal administration. However, when administered orally, A-784168, with good CNS penetration, was much more potent than A-795614. Together, these results demonstrate that TRPV1 receptors in the CNS play an important role in pain mediated by central sensitization. In addition, these results demonstrate that significant CNS penetration is necessary for a TRPV1 antagonist to produce broad-spectrum analgesia.
Figures

Similar articles
-
(R)-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(1H-indazol-4-yl)-urea (ABT-102) blocks polymodal activation of transient receptor potential vanilloid 1 receptors in vitro and heat-evoked firing of spinal dorsal horn neurons in vivo.J Pharmacol Exp Ther. 2008 Sep;326(3):879-88. doi: 10.1124/jpet.108.138511. Epub 2008 May 30. J Pharmacol Exp Ther. 2008. PMID: 18515644
-
Additive antinociceptive effects of the selective Nav1.8 blocker A-803467 and selective TRPV1 antagonists in rat inflammatory and neuropathic pain models.J Pain. 2009 Mar;10(3):306-15. doi: 10.1016/j.jpain.2008.09.007. Epub 2008 Dec 13. J Pain. 2009. PMID: 19070548
-
Differential involvement of TRPV1 receptors at the central and peripheral nerves in CFA-induced mechanical and thermal hyperalgesia.J Pharm Pharmacol. 2007 May;59(5):733-8. doi: 10.1211/jpp.59.5.0015. J Pharm Pharmacol. 2007. PMID: 17524240
-
TRPV1 antagonists as a potential treatment for hyperalgesia.Recent Pat CNS Drug Discov. 2006 Jan;1(1):65-76. doi: 10.2174/157488906775245309. Recent Pat CNS Drug Discov. 2006. PMID: 18221192 Review.
-
Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks.Brain Res Rev. 2009 Apr;60(1):267-77. doi: 10.1016/j.brainresrev.2008.12.006. Epub 2008 Dec 25. Brain Res Rev. 2009. PMID: 19150372 Review.
Cited by
-
The discovery and development of analgesics: new mechanisms, new modalities.J Clin Invest. 2010 Nov;120(11):3753-9. doi: 10.1172/JCI43195. Epub 2010 Nov 1. J Clin Invest. 2010. PMID: 21041957 Free PMC article. Review.
-
Regulation of Membrane Calcium Transport Proteins by the Surrounding Lipid Environment.Biomolecules. 2019 Sep 20;9(10):513. doi: 10.3390/biom9100513. Biomolecules. 2019. PMID: 31547139 Free PMC article. Review.
-
Targeting Pain-evoking Transient Receptor Potential Channels for the Treatment of Pain.Curr Neuropharmacol. 2013 Dec;11(6):652-63. doi: 10.2174/1570159X113119990040. Curr Neuropharmacol. 2013. PMID: 24396340 Free PMC article.
-
An update on targets for treating osteoarthritis pain: NGF and TRPV1.Curr Treatm Opt Rheumatol. 2020 Sep;6(3):129-145. doi: 10.1007/s40674-020-00146-x. Epub 2020 May 6. Curr Treatm Opt Rheumatol. 2020. PMID: 34178580 Free PMC article.
-
TRPV1: a stress response protein in the central nervous system.Am J Neurodegener Dis. 2012;1(1):1-14. Epub 2012 Apr 1. Am J Neurodegener Dis. 2012. PMID: 22737633 Free PMC article.
References
-
- Amaya F, Shimosato G, Nagano M, Ueda M, Hashimoto S, Tanaka Y, Suzuki H, Tanaka M. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur J Neurosci. 2004;20:2303–2310. - PubMed
-
- Bianchi BR, Lee CH, Jarvis MF, El Kouhen R, Moreland RB, Faltynek CR, Puttfarken PS. Modulation of human TRPV1 receptor activity by extracellular proteins and host cell expression system. Eur J Pharmacol. 2006;537:20–30. - PubMed
-
- Blair HC, Teitelbaum SL, Ghiselli R, Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989;245:855–857. - PubMed
-
- Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarth Cartil. 2003;11:821–830. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
