Receptor and transmitter release properties set the time course of retinal inhibition
- PMID: 16971525
- PMCID: PMC6674600
- DOI: 10.1523/JNEUROSCI.2591-06.2006
Receptor and transmitter release properties set the time course of retinal inhibition
Abstract
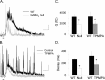
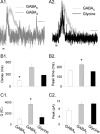
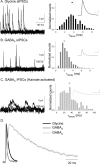
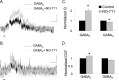
Synaptic inhibition is determined by the properties of postsynaptic receptors, neurotransmitter release, and clearance, but little is known about how these factors shape sensation-evoked inhibition. The retina is an ideal system to investigate inhibition because it can be activated physiologically with light, and separate inhibitory pathways can be assayed by recording from rod bipolar cells that possess distinct glycine, GABA(A), and GABA(C) receptors (R). We show that receptor properties differentially shape spontaneous IPSCs, whereas both transmitter release and receptor properties shape light-evoked (L) IPSCs. GABA(C)R-mediated IPSCs decayed the slowest, whereas glycineR- and GABA(A)R-mediated IPSCs decayed more rapidly. Slow GABA(C)Rs determined the L-IPSC decay, whereas GABA(A)Rs and glycineRs, which mediated rapid onset responses, determined the start of the L-IPSC. Both fast and slow inhibitory inputs distinctly shaped the output of rod bipolar cells. The slow GABA(C)Rs truncated glutamate release, making the A17 amacrine cell L-EPSCs more transient, whereas the fast GABA(A)R and glycineRs reduced the initial phase of glutamate release, limiting the peak amplitude of the L-EPSC. Estimates of transmitter release time courses suggested that glycine release was more prolonged than GABA release. The time course of GABA release activating GABA(C)Rs was slower than that activating GABA(A)Rs, consistent with spillover activation of GABA(C)Rs. Thus, both postsynaptic receptor and transmitter release properties shape light-evoked inhibition in retina.
Figures






Similar articles
-
GABA(A), GABA(C) and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells.J Physiol. 2006 Apr 1;572(Pt 1):215-25. doi: 10.1113/jphysiol.2005.103648. Epub 2006 Jan 26. J Physiol. 2006. PMID: 16439422 Free PMC article.
-
Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina.J Physiol. 2007 Jul 15;582(Pt 2):569-82. doi: 10.1113/jphysiol.2007.131763. Epub 2007 Apr 26. J Physiol. 2007. PMID: 17463042 Free PMC article.
-
Different types of retinal inhibition have distinct neurotransmitter release properties.J Neurophysiol. 2015 Apr 1;113(7):2078-90. doi: 10.1152/jn.00447.2014. Epub 2015 Jan 7. J Neurophysiol. 2015. PMID: 25568157 Free PMC article.
-
Receptor targets of amacrine cells.Vis Neurosci. 2012 Jan;29(1):11-29. doi: 10.1017/S0952523812000028. Vis Neurosci. 2012. PMID: 22310370 Free PMC article. Review.
-
The enigma of transmitter-selective receptor accumulation at developing inhibitory synapses.Cell Tissue Res. 2003 Mar;311(3):271-6. doi: 10.1007/s00441-002-0694-9. Epub 2003 Feb 11. Cell Tissue Res. 2003. PMID: 12658435 Review.
Cited by
-
Retinal ON bipolar cells express a new PCP2 splice variant that accelerates the light response.J Neurosci. 2008 Sep 3;28(36):8873-84. doi: 10.1523/JNEUROSCI.0812-08.2008. J Neurosci. 2008. PMID: 18768681 Free PMC article.
-
Relief of Mg²⁺-dependent inhibition of TRPM1 by PKCα at the rod bipolar cell synapse.J Neurosci. 2011 Sep 21;31(38):13596-603. doi: 10.1523/JNEUROSCI.2655-11.2011. J Neurosci. 2011. PMID: 21940450 Free PMC article.
-
Synaptic inhibition tunes contrast computation in the retina.Vis Neurosci. 2019 Jan;36:E006. doi: 10.1017/S095252381900004X. Vis Neurosci. 2019. PMID: 31199207 Free PMC article.
-
Layers of inhibitory networks shape receptive field properties of AII amacrine cells.Cell Rep. 2023 Nov 28;42(11):113390. doi: 10.1016/j.celrep.2023.113390. Epub 2023 Nov 5. Cell Rep. 2023. PMID: 37930888 Free PMC article.
-
Differential encoding of spatial information among retinal on cone bipolar cells.J Neurophysiol. 2015 Sep;114(3):1757-72. doi: 10.1152/jn.00287.2015. Epub 2015 Jul 22. J Neurophysiol. 2015. PMID: 26203104 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
