Endothelial proliferation and increased blood-brain barrier permeability in the basal ganglia in a rat model of 3,4-dihydroxyphenyl-L-alanine-induced dyskinesia
- PMID: 16971529
- PMCID: PMC6674611
- DOI: 10.1523/JNEUROSCI.0944-06.2006
Endothelial proliferation and increased blood-brain barrier permeability in the basal ganglia in a rat model of 3,4-dihydroxyphenyl-L-alanine-induced dyskinesia
Abstract
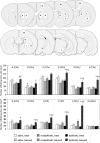
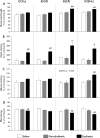
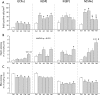
3,4-Dihydroxyphenyl-L-alanine (L-DOPA)-induced dyskinesia is associated with molecular and synaptic plasticity in the basal ganglia, but the occurrence of structural remodeling through cell genesis has not been explored. In this study, rats with 6-hydroxydopamine lesions received injections of the thymidine analog 5-bromo-2'-deoxyuridine (BrdU) concomitantly with L-DOPA for 2 weeks. A large number of BrdU-positive cells were found in the striatum and its output structures (globus pallidus, entopeduncular nucleus, and substantia nigra pars reticulata) in L-DOPA-treated rats that had developed dyskinesia. The vast majority (60-80%) of the newborn cells stained positively for endothelial markers. This endothelial proliferation was associated with an upregulation of immature endothelial markers (nestin) and a downregulation of endothelial barrier antigen on blood vessel walls. In addition, dyskinetic rats exhibited a significant increase in total blood vessel length and a visible extravasation of serum albumin in the two structures in which endothelial proliferation was most pronounced (substantia nigra pars reticulata and entopeduncular nucleus). The present study provides the first evidence of angiogenesis and blood-brain barrier dysfunction in an experimental model of L-DOPA-induced dyskinesia. These microvascular changes are likely to affect the kinetics of L-DOPA entry into the brain, favoring the occurrence of motor complications.
Figures






Similar articles
-
Impact of L-DOPA treatment on regional cerebral blood flow and metabolism in the basal ganglia in a rat model of Parkinson's disease.Neuroimage. 2012 May 15;61(1):228-39. doi: 10.1016/j.neuroimage.2012.02.066. Epub 2012 Mar 3. Neuroimage. 2012. PMID: 22406356 Free PMC article.
-
Vascular endothelial growth factor is upregulated by L-dopa in the parkinsonian brain: implications for the development of dyskinesia.Brain. 2011 Aug;134(Pt 8):2339-57. doi: 10.1093/brain/awr165. Epub 2011 Jul 19. Brain. 2011. PMID: 21771855 Free PMC article.
-
Differential involvement of D1 and D2 dopamine receptors in L-DOPA-induced angiogenic activity in a rat model of Parkinson's disease.Neuropsychopharmacology. 2009 Nov;34(12):2477-88. doi: 10.1038/npp.2009.74. Epub 2009 Jul 15. Neuropsychopharmacology. 2009. PMID: 19606087
-
Post- versus presynaptic plasticity in L-DOPA-induced dyskinesia.J Neurochem. 2006 Oct;99(2):381-92. doi: 10.1111/j.1471-4159.2006.04124.x. Epub 2006 Aug 29. J Neurochem. 2006. PMID: 16942598 Review.
-
Plastic effects of L-DOPA treatment in the basal ganglia and their relevance to the development of dyskinesia.Parkinsonism Relat Disord. 2009 Dec;15 Suppl 3:S59-63. doi: 10.1016/S1353-8020(09)70782-5. Parkinsonism Relat Disord. 2009. PMID: 20083009 Review.
Cited by
-
Functional neuroimaging in Parkinson's disease.Cold Spring Harb Perspect Med. 2012 May;2(5):a009274. doi: 10.1101/cshperspect.a009274. Cold Spring Harb Perspect Med. 2012. PMID: 22553499 Free PMC article. Review.
-
Metabolic brain networks in translational neurology: concepts and applications.Ann Neurol. 2012 Nov;72(5):635-47. doi: 10.1002/ana.23631. Epub 2012 Aug 31. Ann Neurol. 2012. PMID: 22941893 Free PMC article. Review.
-
Heterogeneity in the rat brain vasculature revealed by quantitative confocal analysis of endothelial barrier antigen and P-glycoprotein expression.J Cereb Blood Flow Metab. 2012 Jan;32(1):81-92. doi: 10.1038/jcbfm.2011.109. Epub 2011 Jul 27. J Cereb Blood Flow Metab. 2012. PMID: 21792241 Free PMC article.
-
Mass spectrometry imaging identifies abnormally elevated brain l-DOPA levels and extrastriatal monoaminergic dysregulation in l-DOPA-induced dyskinesia.Sci Adv. 2021 Jan 6;7(2):eabe5948. doi: 10.1126/sciadv.abe5948. Print 2021 Jan. Sci Adv. 2021. PMID: 33523980 Free PMC article.
-
Substance P and its tachykinin NK1 receptor: a novel neuroprotective target for Parkinson's disease.Neural Regen Res. 2015 Sep;10(9):1403-5. doi: 10.4103/1673-5374.165505. Neural Regen Res. 2015. PMID: 26604896 Free PMC article. No abstract available.
References
-
- Andersson M, Hilbertson A, Cenci MA. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol Dis. 1999;6:461–474. - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
-
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. - PubMed
-
- Barcia C, Bautista V, Sanchez-Bahillo A, Fernandez-Villalba E, Faucheux B, Poza YPM, Fernandez Barreiro A, Hirsch EC, Herrero MT. Changes in vascularization in substantia nigra pars compacta of monkeys rendered parkinsonian. J Neural Transm. 2005;112:1237–1248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
