Expression of multiple P2X receptors by glossopharyngeal neurons projecting to rat carotid body O2-chemoreceptors: role in nitric oxide-mediated efferent inhibition
- PMID: 16971532
- PMCID: PMC6674604
- DOI: 10.1523/JNEUROSCI.1672-06.2006
Expression of multiple P2X receptors by glossopharyngeal neurons projecting to rat carotid body O2-chemoreceptors: role in nitric oxide-mediated efferent inhibition
Abstract
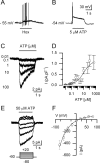
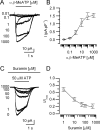
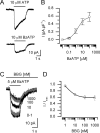
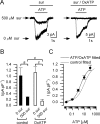
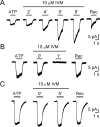
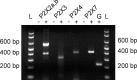
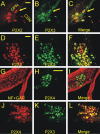
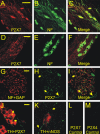
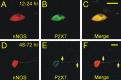
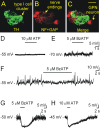
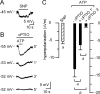
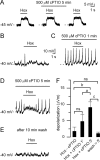
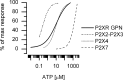
In mammals, ventilation is peripherally controlled by the carotid body (CB), which receives afferent innervation from the petrosal ganglion and efferent innervation from neurons located along the glossopharyngeal nerve (GPN). GPN neurons give rise to the "efferent inhibitory" pathway via a plexus of neuronal nitric oxide (NO) synthase-positive fibers, believed to be responsible for CB chemoreceptor inhibition via NO release. Although NO is elevated during natural CB stimulation by hypoxia, the underlying mechanisms are unclear. We hypothesized that ATP, released by rat CB chemoreceptors (type 1 cells) and/or red blood cells during hypoxia, may directly activate GPN neurons and contribute to NO-mediated inhibition. Using combined electrophysiological, molecular, and confocal immunofluorescence techniques, we detected the expression of multiple P2X receptors in GPN neurons. These receptors involve at least four different purinergic subunits: P2X2 [and the splice variant P2X2(b)], P2X3, P2X4, and P2X7. Using a novel coculture preparation of CB type I cell clusters and GPN neurons, we tested the role of P2X signaling on CB function. In cocultures, fast application of ATP, or its synthetic analog 2',3'-O-(4 benzoylbenzoyl)-ATP, caused type I cell hyperpolarization that was prevented in the presence of the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide potassium. These data suggest that ATP released during hypoxic stress from CB chemoreceptors (and/or red blood cells) will cause GPN neuron depolarization mediated by multiple P2X receptors. Activation of this pathway will lead to calcium influx and efferent inhibition of CB chemoreceptors via NO synthesis and consequent release.
Figures
References
-
- Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E, Jarvis MF, van Biesen T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. - PubMed
-
- Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett. 1995;375:129–133. - PubMed
-
- Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. - PubMed
-
- Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. - PubMed
-
- Buttigieg J, Nurse CA. Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochem Biophys Res Commun. 2004;322:82–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
