Odorant deprivation reversibly modulates transsynaptic changes in the NR2B-mediated CREB pathway in mouse piriform cortex
- PMID: 16971539
- PMCID: PMC6674609
- DOI: 10.1523/JNEUROSCI.1727-06.2006
Odorant deprivation reversibly modulates transsynaptic changes in the NR2B-mediated CREB pathway in mouse piriform cortex
Abstract
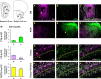
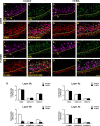
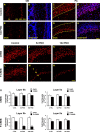
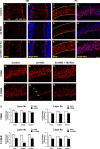
The olfactory system is an outstanding model for understanding activity-dependent neuronal plasticity in mammals. Olfactory sensory neurons (OSNs) in the periphery project onto mitral/tufted cells in the olfactory bulb (OB) and these mitral/tufted cells in turn project to piriform cortex (PC). Numerous studies have examined changes in OB after a permanent OSN ablation, but little is known about "trans-transsynaptic" changes taking place in the PC. Permanent zinc sulfate lesion of the olfactory epithelium resulted in a selective loss of the NMDA receptor NR2B protein and mRNA expression in pyramidal cells in layer IIb of PC after 2-7 d. Regulatory elements affected by NR2B signaling, namely the phosphorylation of CREB, were also downregulated only in layer IIb neurons. These changes could be caused by OSN axon loss in the zinc sulfate lesion, or to a reduced activity. To test this hypothesis, we performed both permanent and reversible naris occlusion, which blocks odorant access to the nasal cavities and OSN activity. The expression of NR2B and phospho-CREB were downregulated 5 d after occlusion and this reduction was fully restored 10 d after reopening of the naris. Subsequently, we identified the subset of pyramidal cells in layer IIb that are especially sensitive to the loss of odor-evoked activity using double retrograde tracers. In summary, the present study provides an initial characterization of the molecular mechanisms associated with odor stimulation on second order neuronal plasticity and phenotype in the olfactory system.
Figures
Similar articles
-
Regulation of c-Fos mRNA and fos protein expression in olfactory bulbs from unilaterally odor-deprived adult mice.Int J Dev Neurosci. 1996 Nov;14(7-8):971-82. doi: 10.1016/s0736-5748(96)00044-5. Int J Dev Neurosci. 1996. PMID: 9010739
-
Differential Effects of Nasal Inflammation and Odor Deprivation on Layer-Specific Degeneration of the Mouse Olfactory Bulb.eNeuro. 2020 Apr 17;7(2):ENEURO.0403-19.2020. doi: 10.1523/ENEURO.0403-19.2020. Print 2020 Mar/Apr. eNeuro. 2020. PMID: 32220858 Free PMC article.
-
Expression of the NMDA receptor subunit GluN3A (NR3A) in the olfactory system and its regulatory role on olfaction in the adult mouse.Brain Struct Funct. 2016 Jul;221(6):3259-73. doi: 10.1007/s00429-015-1099-3. Epub 2015 Sep 3. Brain Struct Funct. 2016. PMID: 26334321
-
Developmental regulation of olfactory circuit formation in mice.Dev Growth Differ. 2020 May;62(4):199-213. doi: 10.1111/dgd.12657. Epub 2020 Feb 28. Dev Growth Differ. 2020. PMID: 32112394 Free PMC article. Review.
-
Olfactory receptor function.Handb Clin Neurol. 2019;164:67-78. doi: 10.1016/B978-0-444-63855-7.00005-8. Handb Clin Neurol. 2019. PMID: 31604564 Review.
Cited by
-
TNF-α Orchestrates Experience-Dependent Plasticity of Excitatory and Inhibitory Synapses in the Anterior Piriform Cortex.Front Neurosci. 2022 Apr 26;16:824454. doi: 10.3389/fnins.2022.824454. eCollection 2022. Front Neurosci. 2022. PMID: 35557610 Free PMC article.
-
Olfactory system modulation of hippocampal cell death.Neurosci Lett. 2007 Jul 5;422(1):13-7. doi: 10.1016/j.neulet.2007.05.041. Epub 2007 Jun 14. Neurosci Lett. 2007. PMID: 17597296 Free PMC article.
-
Functional deprivation promotes amyloid plaque pathogenesis in Tg2576 mouse olfactory bulb and piriform cortex.Eur J Neurosci. 2010 Feb;31(4):710-21. doi: 10.1111/j.1460-9568.2010.07103.x. Eur J Neurosci. 2010. PMID: 20384814 Free PMC article.
-
Is brain amyloid production a cause or a result of dementia of the Alzheimer's type?J Alzheimers Dis. 2010;22(2):393-9. doi: 10.3233/JAD-2010-100846. J Alzheimers Dis. 2010. PMID: 20847431 Free PMC article. Review.
-
Age-related impairments in memory and in CREB and pCREB expression in hippocampus and amygdala following inhibitory avoidance training.Mech Ageing Dev. 2012 May;133(5):291-9. doi: 10.1016/j.mad.2012.03.004. Epub 2012 Mar 16. Mech Ageing Dev. 2012. PMID: 22445851 Free PMC article.
References
-
- Bading H, Greenberg ME. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991;253:912–914. - PubMed
-
- Baker H, Towle AC, Margolis FL. Differential afferent regulation of dopaminergic and GABAergic neurons in the mouse main olfactory bulb. Brain Res. 1988;450:69–80. - PubMed
-
- Baker H, Morel K, Stone DM, Maruniak JA. Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res. 1993;614:109–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
