Postreactivation glucocorticoids impair recall of established fear memory
- PMID: 16971540
- PMCID: PMC3917138
- DOI: 10.1523/JNEUROSCI.2397-06.2006
Postreactivation glucocorticoids impair recall of established fear memory
Abstract
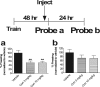
Pavlovian fear conditioning provides one of the best rodent models of acquired anxiety disorders, including posttraumatic stress disorder. Injection of a variety of drugs after training in fear-conditioning paradigms can impair consolidation of fear memories. Indeed, early clinical trials suggest that immediate administration of such drugs after a traumatic event may decrease the risk of developing posttraumatic stress disorder in humans (Pitman et al., 2002; Vaiva et al., 2003). The use of such a treatment is limited by the difficulty of treating every patient at risk and by the difficulty in predicting which patients will experience chronic adverse consequences. Recent clinical trials suggest that administration of glucocorticoids may have a beneficial effect on established posttraumatic stress disorder (Aerni et al., 2004) and specific phobia (Soravia et al., 2006). Conversely, glucocorticoid administration after training is known to enhance memory consolidation (McGaugh and Roozendaal, 2002; Roozendaal, 2002). From a clinical perspective, enhancement of a fear memory or a reactivated fear memory would not be desirable. We report here that when glucocorticoids are administered immediately after reactivation of a contextual fear memory, subsequent recall is significantly diminished. Additional experiments support the interpretation that glucocorticoids not only decrease fear memory retrieval but, in addition, augment consolidation of fear memory extinction rather than decreasing reconsolidation. These findings provide a rodent model for a potential treatment of established acquired anxiety disorders in humans, as suggested by others (Aerni et al., 2004; Schelling et al., 2004), based on a mechanism of enhanced extinction.
Figures



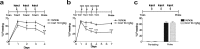
References
-
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–187. - PubMed
-
- Abercrombie HC, Kalin NH, Davidson RJ. Acute cortisol elevations cause heightened arousal ratings of objectively nonarousing stimuli. Emotion. 2005;5:354–359. - PubMed
-
- Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, Nitsch RM, Schnyder U, de Quervain DJ. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004;161:1488–1490. - PubMed
-
- Anokhin KV, Tiunova AA, Rose SP. Reminder effects–reconsolidation or retrieval deficit? Pharmacological dissection with protein synthesis inhibitors following reminder for a passive-avoidance task in young chicks. Eur J Neurosci. 2002;15:1759–1765. - PubMed
-
- Barrett D, Gonzalez-Lima F. Behavioral effects of metyrapone on pavlovian extinction. Neurosci Lett. 2004;371:91–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
