Stranger in a strange land
- PMID: 16972895
- PMCID: PMC1637092
- DOI: 10.1111/j.1600-065X.2006.00436.x
Stranger in a strange land
Abstract
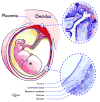
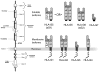
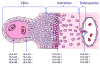
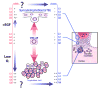
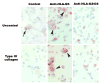
Mammalian mothers and their embryos/fetuses are almost invariably genetically different, which raises the question of how the mother's immune system is diverted so as to permit cohabitation with the 'foreign' body. Several decades of research have shown that multiple cooperative systems sanction uteroplacental immune privilege. These systems include production of several varieties of soluble immunosuppressive molecules in the uterus and the placenta and strict regulation of the molecules expressed on or by placental trophoblast cells. Trophoblast, a unique lineage without counterpart in adult tissues, is in direct contact with maternal blood and tissue. The major graft rejection-promoting molecules, human leukocyte antigens (HLAs), are tightly regulated in these cells, with none of HLA-A, HLA-B, or HLA class II antigens expressed. The HLA class Ib antigens, HLA-E, HLA-F, and HLA-G, are detectable on some subpopulations. Our studies have focused on the expression, regulation, and functions of the soluble isoforms of HLA-G, which circulate in maternal blood and are present at high levels in the pregnant uterus. These isoforms are derived from the single HLA-G gene by alternative splicing and are now known to have immunosuppressive properties. Ours and other studies indicate that soluble HLA-G proteins may comprise a unique tolerogenic system for establishing local immune privilege during pregnancy.
Figures


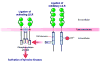

References
-
- Heinlein RA. New York: GP Putnam’s Sons; 1961. Stranger in a Strange Land.
-
- Burton GJ, Hempstock J, Jauniaux E. Oxygen, early embryonic metabolism and free radical-mediated embryopathies. Reprod Biomed Online. 2002;6:84–96. - PubMed
-
- Bulmer JN, Pace D, Ritson A. Immunoregulatory cells in human decidua: morphology, immunohistochemistry and function. Reprod Nutr Dev. 1988;28:1599–1613. - PubMed
-
- Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. - PubMed
-
- Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19:681–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

