Mendelian resistance to human norovirus infections
- PMID: 16973373
- PMCID: PMC7129677
- DOI: 10.1016/j.smim.2006.07.009
Mendelian resistance to human norovirus infections
Abstract
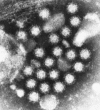
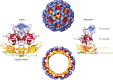
Noroviruses have emerged as a major cause of acute gastroenteritis in humans of all ages. Despite high infectivity of the virus and lack of long-term immunity, volunteer and authentic studies has suggested the existence of inherited protective factors. Recent studies have shown that histo-blood group antigens (HBGAs) and in particular secretor status controlled by the alpha1,2fucosyltransferase FUT2 gene determine susceptibility to norovirus infections, with nonsecretors (FUT2-/-), representing 20% of Europeans, being highly resistant to symptomatic infections with major strains of norovirus. Moreover, the capsid protein from distinct strains shows different HBGA specificities, suggesting a host-pathogen co-evolution driven by carbohydrate-protein interactions.
Figures


References
-
- Brown K.E., Hibbs J.R., Gallinella G. Resistance to parvovirus B19 infection due to lack of virus receptor (erythrocyte P antigen) N Engl J Med. 1994;330:1192–1196. - PubMed
-
- Kindberg E., Hejdeman B., Bratt G. A nonsense mutation (428G → A) in the fucosyltransferase FUT2 gene affects the progression of HIV-1 infection. AIDS. 2006;20:685–689. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

