ORF18 is a transfactor that is essential for late gene transcription of a gammaherpesvirus
- PMID: 16973577
- PMCID: PMC1617240
- DOI: 10.1128/JVI.00246-06
ORF18 is a transfactor that is essential for late gene transcription of a gammaherpesvirus
Abstract
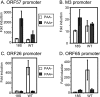
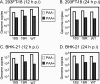
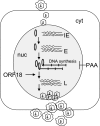
Lytic replication of the tumor-associated human gammaherpesviruses Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus has important implications in pathogenesis and tumorigenesis. Herpesvirus lytic genes have been temporally classified as exhibiting immediate-early (IE), early, and late expression kinetics. Though the regulation of IE and early gene expression has been studied extensively, very little is known regarding the regulation of late gene expression. Late genes, which primarily encode virion structural proteins, require viral DNA replication for their expression. We have identified a murine gammaherpesvirus 68 (MHV-68) early lytic gene, ORF18, essential for viral replication. ORF18 is conserved in both beta- and gammaherpesviruses. By generating an MHV-68 ORF18-null virus, we characterized the stage of the virus lytic cascade that requires the function of ORF18. Gene expression profiling and quantitation of viral DNA synthesis of the ORF18-null virus revealed that the expression of early genes and viral DNA replication were not affected; however, the transcription of late genes was abolished. Hence, we have identified a gammaherpesvirus-encoded factor essential for the expression of late genes independently of viral DNA synthesis.
Figures








References
-
- AuCoin, D. P., K. S. Colletti, S. A. Cei, I. Papouskova, M. Tarrant, and G. S. Pari. 2004. Amplification of the Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 lytic origin of DNA replication is dependent upon a cis-acting AT-rich region and an ORF50 response element and the trans-acting factors ORF50 (K-Rta) and K8 (K-bZIP). Virology 318:542-555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

