The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9
- PMID: 16973587
- PMCID: PMC1617255
- DOI: 10.1128/JVI.00878-06
The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9
Abstract
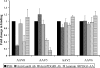
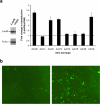
Adeno-associated virus serotype 8 (AAV8) is currently emerging as a powerful gene transfer vector, owing to its capability to efficiently transduce many different tissues in vivo. While this is believed to be in part due to its ability to uncoat more readily than other AAV serotypes such as AAV2, understanding all the processes behind AAV8 transduction is important for its application and optimal use in human gene therapy. Here, we provide the first report of a cellular receptor for AAV8, the 37/67-kDa laminin receptor (LamR). We document binding of LamR to AAV8 capsid proteins and intact virions in vitro and demonstrate its contribution to AAV8 transduction of cultured cells and mouse liver in vivo. We also show that LamR plays a role in transduction by three other closely related serotypes (AAV2, -3, and -9). Sequence and deletion analysis allowed us to map LamR binding to two protein subdomains predicted to be exposed on the AAV capsid exterior. Use of LamR, which is constitutively expressed in many clinically relevant tissues and is overexpressed in numerous cancers, provides a molecular explanation for AAV8's broad tissue tropism. Along with its robust transduction efficiency, our findings support the continued development of AAV8-based vectors for clinical applications in humans, especially for tumor gene therapy.
Figures
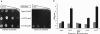

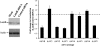
References
-
- Ardini, E., B. Sporchia, L. Pollegioni, M. Modugno, C. Ghirelli, F. Castiglioni, E. Tagliabue, and S. Menard. 2002. Identification of a novel function for 67-kDa laminin receptor: increase in laminin degradation rate and release of motility fragments. Cancer Res. 62:1321-1325. - PubMed
-
- Ardini, E., E. Tagliabue, A. Magnifico, S. Buto, V. Castronovo, M. I. Colnaghi, and S. Menard. 1997. Co-regulation and physical association of the 67-kDa monomeric laminin receptor and the α6β4 integrin. J. Biol. Chem. 272:2342-2345. - PubMed
-
- Blackburn, S. D., R. A. Steadman, and F. B. Johnson. 2006. Attachment of adeno-associated virus type 3H to fibroblast growth factor receptor 1. Arch. Virol. 151:617-623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

