Cell physiology of cAMP sensor Epac
- PMID: 16973695
- PMCID: PMC2000694
- DOI: 10.1113/jphysiol.2006.119644
Cell physiology of cAMP sensor Epac
Abstract
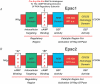
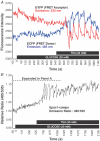
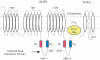
Epac is an acronym for the exchange proteins activated directly by cyclic AMP, a family of cAMP-regulated guanine nucleotide exchange factors (cAMPGEFs) that mediate protein kinase A (PKA)-independent signal transduction properties of the second messenger cAMP. Two variants of Epac exist (Epac1 and Epac2), both of which couple cAMP production to the activation of Rap, a small molecular weight GTPase of the Ras family. By activating Rap in an Epac-mediated manner, cAMP influences diverse cellular processes that include integrin-mediated cell adhesion, vascular endothelial cell barrier formation, and cardiac myocyte gap junction formation. Recently, the identification of previously unrecognized physiological processes regulated by Epac has been made possible by the development of Epac-selective cyclic AMP analogues (ESCAs). These cell-permeant analogues of cAMP activate both Epac1 and Epac2, whereas they fail to activate PKA when used at low concentrations. ESCAs such as 8-pCPT-2'-O-Me-cAMP and 8-pMeOPT-2'-O-Me-cAMP are reported to alter Na(+), K(+), Ca(2+) and Cl(-) channel function, intracellular [Ca(2+)], and Na(+)-H(+) transporter activity in multiple cell types. Moreover, new studies examining the actions of ESCAs on neurons, pancreatic beta cells, pituitary cells and sperm demonstrate a major role for Epac in the stimulation of exocytosis by cAMP. This topical review provides an update concerning novel PKA-independent features of cAMP signal transduction that are likely to be Epac-mediated. Emphasized is the emerging role of Epac in the cAMP-dependent regulation of ion channel function, intracellular Ca(2+) signalling, ion transporter activity and exocytosis.
Figures

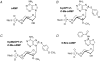

Similar articles
-
Epac-selective cAMP analogs: new tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors.Cell Signal. 2008 Jan;20(1):10-20. doi: 10.1016/j.cellsig.2007.07.009. Epub 2007 Jul 25. Cell Signal. 2008. PMID: 17716863 Free PMC article. Review.
-
cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic beta cells and rat INS-1 cells.J Physiol. 2006 Jun 15;573(Pt 3):595-609. doi: 10.1113/jphysiol.2006.107391. Epub 2006 Apr 13. J Physiol. 2006. PMID: 16613879 Free PMC article.
-
Epac: effectors and biological functions.Naunyn Schmiedebergs Arch Pharmacol. 2008 Jun;377(4-6):345-57. doi: 10.1007/s00210-007-0246-7. Epub 2008 Jan 5. Naunyn Schmiedebergs Arch Pharmacol. 2008. PMID: 18176800 Review.
-
Role of the cAMP sensor Epac as a determinant of KATP channel ATP sensitivity in human pancreatic beta-cells and rat INS-1 cells.J Physiol. 2008 Mar 1;586(5):1307-19. doi: 10.1113/jphysiol.2007.143818. Epub 2008 Jan 17. J Physiol. 2008. PMID: 18202100 Free PMC article.
-
cAMP signalling protects proximal tubular epithelial cells from cisplatin-induced apoptosis via activation of Epac.Br J Pharmacol. 2012 Feb;165(4b):1137-50. doi: 10.1111/j.1476-5381.2011.01594.x. Br J Pharmacol. 2012. PMID: 21745194 Free PMC article.
Cited by
-
Cyclic Adenosine Monophosphate-Mediated Enhancement of Vascular Endothelial Growth Factor Released by Differentiated Human Monocytic Cells: The Role of Protein Kinase A.Med Princ Pract. 2015;24(6):548-54. doi: 10.1159/000433540. Epub 2015 Jul 1. Med Princ Pract. 2015. PMID: 26139101 Free PMC article.
-
Identification and function of exchange proteins activated directly by cyclic AMP (Epac) in mammalian spermatozoa.PLoS One. 2012;7(5):e37713. doi: 10.1371/journal.pone.0037713. Epub 2012 May 25. PLoS One. 2012. PMID: 22662198 Free PMC article.
-
Targeting beta-cell mass in type 2 diabetes: promise and limitations of new drugs based on incretins.Endocr Rev. 2008 May;29(3):367-79. doi: 10.1210/er.2007-0031. Epub 2008 Feb 21. Endocr Rev. 2008. PMID: 18292465 Free PMC article. Review.
-
Restoration of Glucose-Stimulated Cdc42-Pak1 Activation and Insulin Secretion by a Selective Epac Activator in Type 2 Diabetic Human Islets.Diabetes. 2018 Oct;67(10):1999-2011. doi: 10.2337/db17-1174. Epub 2018 Jul 9. Diabetes. 2018. PMID: 29986926 Free PMC article.
-
Cilostazol Induces PGI2 Production via Activation of the Downstream Epac-1/Rap1 Signaling Cascade to Increase Intracellular Calcium by PLCε and to Activate p44/42 MAPK in Human Aortic Endothelial Cells.PLoS One. 2015 Jul 16;10(7):e0132835. doi: 10.1371/journal.pone.0132835. eCollection 2015. PLoS One. 2015. PMID: 26181635 Free PMC article.
References
-
- Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP. J Immunol. 2005;174:595–599. - PubMed
-
- Barg S, Huang P, Eliasson L, Nelson DJ, Obermuller S, Rorsman P, Thevenod F, Renstrom E. Priming of insulin granules for exocytosis by granular Cl− uptake and acidification. J Cell Sci. 2001;114:2145–2154. - PubMed
-
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. - PubMed
-
- Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

