A delayed gonadotropin-dependent and growth factor-mediated activation of the extracellular signal-regulated kinase 1/2 cascade negatively regulates aromatase expression in granulosa cells
- PMID: 16973759
- PMCID: PMC1665466
- DOI: 10.1210/me.2006-0241
A delayed gonadotropin-dependent and growth factor-mediated activation of the extracellular signal-regulated kinase 1/2 cascade negatively regulates aromatase expression in granulosa cells
Abstract
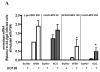
Human chorionic gonadotropin and human FSH (hFSH) elicit a transient increase in ERK1/2 phosphorylation lasting less than 60 min in immature granulosa cells expressing a low density of gonadotropin receptors. In cells expressing a high density of receptors, human chorionic gonadotropin and human FSH elicit this fast transient increase in ERK1/2 phosphorylation and also a delayed and more sustained increase that is detectable after 6-9 h. Both the early and delayed increases in ERK1/2 phosphorylation can be blocked with inhibitors of protein kinase A, the epidermal growth factor receptor kinase, metalloproteases, and MAPK kinase. The delayed effect, but not the early effect, can also be blocked with an inhibitor of protein kinase C. Because the delayed increase in ERK1/2 phosphorylation correlates with low aromatase expression in response to gonadotropins, we tested the effects of these inhibitors on aromatase expression. These inhibitors had little or no effect on gonadotropin-induced aromatase expression in cells expressing a low density of receptors, but they enhanced gonadotropin-induced aromatase expression in cells expressing a high density of receptors. Phorbol esters also induced a prolonged increase in ERK1/2 phosphorylation and, when added together with hFSH, blocked the induction of aromatase expression by hFSH in cells expressing a low density of hFSH receptor. A MAPK kinase inhibitor reversed the inhibitory effect of the phorbol ester on aromatase induction. We conclude that the effects of gonadotropins on ERK1/2 phosphorylation are mediated by epidermal growth factor-like growth factors and that the delayed effect is partially mediated by protein kinase C and acts as a negative regulator of aromatase expression.
Figures








References
-
- Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annual Reviews of Biochemistry. 1981;50:465–495. - PubMed
-
- Pierce JG. Gonadotropins: chemistry and biosynthesis. In: Knobil E, Neill JD, Ewing LL, Greenwald GS, Markert CL, Pfaff DW, editors. The physiology of reproduction. New York: Raven Press; 1988. pp. 1335–1348.
-
- Vassart G, Pardo L, Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci. 2004;29:119–126. - PubMed
-
- Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology and pathophysiology. Endocr Rev. 1997;18:739–773. - PubMed
-
- Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21:551–583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

