High gamma power is phase-locked to theta oscillations in human neocortex
- PMID: 16973878
- PMCID: PMC2628289
- DOI: 10.1126/science.1128115
High gamma power is phase-locked to theta oscillations in human neocortex
Abstract
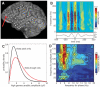
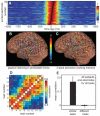
We observed robust coupling between the high- and low-frequency bands of ongoing electrical activity in the human brain. In particular, the phase of the low-frequency theta (4 to 8 hertz) rhythm modulates power in the high gamma (80 to 150 hertz) band of the electrocorticogram, with stronger modulation occurring at higher theta amplitudes. Furthermore, different behavioral tasks evoke distinct patterns of theta/high gamma coupling across the cortex. The results indicate that transient coupling between low- and high-frequency brain rhythms coordinates activity in distributed cortical areas, providing a mechanism for effective communication during cognitive processing in humans.
Figures
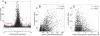
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

