Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-A and transforming growth factor-beta2
- PMID: 16973908
- PMCID: PMC2810464
- DOI: 10.1161/01.RES.0000245188.41002.2c
Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-A and transforming growth factor-beta2
Abstract
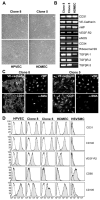
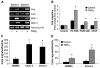
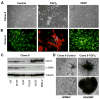
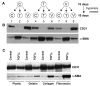
In situ analysis of fetal semilunar valve leaflets has revealed cells coexpressing endothelial and mesenchymal markers along the endothelium, with diminished frequency seen in adult valves. To determine whether such cells are progenitor cells, we isolated clonal populations from human pulmonary valves. The clones expressed endothelial markers but showed potential to further differentiate into endothelium in response to vascular endothelial growth factor (VEGF)-A. When exposed to transforming growth factor (TGF)-beta2, individual clones adopted a mesenchymal phenotype to varying degrees and expressed markers of endothelial to mesenchymal transformation (EMT). Both VEGF- and TGFbeta2-induced phenotypic changes were partially reversible, indicating the plasticity of these cells. When challenged with VEGF or TGFbeta2, a hierarchy of endothelial/mesenchymal potential could be seen among the clonal populations: cells initially closer to an endothelial phenotype showed a strong response to TGFbeta2 that could be inhibited by VEGF, whereas cells closer to a mesenchymal phenotype responded to TGFbeta2 but were resistant to endothelial-inducing effects of VEGF. These findings suggest the presence of bipotential valve progenitor cells with ability to differentiate into either endothelial or interstitial cells of the valve leaflet. Understanding the differentiation potential and function of these cells may be important for understanding heart valve disease and may also be applied to current paradigms for creating tissue-engineered heart valves.
Figures



References
-
- Rabkin-Aikawa E, Mayer JE, Jr, Schoen FJ. Heart valve regeneration. Adv Biochem Eng Biotechnol. 2005;94:141–79. - PubMed
-
- Markwald RR, Fitzharris TP, Manasek FJ. Structural development of endocardial cushions. Am J Anat. 1977;148:85–119. - PubMed
-
- Bernanke DH, Markwald RR. Migratory behavior of cardiac cushion tissue cells in a collagen-lattice culture system. Dev Biol. 1982;91:235–45. - PubMed
-
- Nakajima Y, Mironov V, Yamagishi T, Nakamura H, Markwald RR. Expression of smooth muscle alpha-actin in mesenchymal cells during formation of avian endocardial cushion tissue: a role for transforming growth factor beta3. Dev Dyn. 1997;209:296–309. - PubMed
-
- Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP) Anat Rec. 2000;258:119–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

