Recombinant vesicular stomatitis virus as an HIV-1 vaccine vector
- PMID: 16977404
- PMCID: PMC7079905
- DOI: 10.1007/s00281-006-0042-3
Recombinant vesicular stomatitis virus as an HIV-1 vaccine vector
Abstract
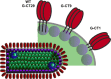
Recombinant vesicular stomatitis virus (rVSV) is currently under evaluation as a human immunodeficiency virus (HIV)-1 vaccine vector. The most compelling reasons to develop rVSV as a vaccine vector include a very low seroprevalence in humans, the ability to infect and robustly express foreign antigens in a broad range of cells, and vigorous growth in continuous cell lines used for vaccine manufacture. Numerous preclinical studies with rVSV vectors expressing antigens from a variety of human pathogens have demonstrated the versatility, flexibility, and potential efficacy of the rVSV vaccine platform. When administered to nonhuman primates (NHPs), rVSV vectors expressing HIV-1 Gag and Env elicited robust HIV-1-specific cellular and humoral immune responses, and animals immunized with rVSV vectors expressing simian immunodeficiency virus (SIV) Gag and HIV Env were protected from AIDS after challenge with a pathogenic SIV/HIV recombinant. However, results from an exploratory neurovirulence study in NHPs indicated that these prototypic rVSV vectors might not be adequately attenuated for widespread use in human populations. To address this safety concern, a variety of different attenuation strategies, designed to produce a range of further attenuated rVSV vectors, are currently under investigation. Additional modifications of further attenuated rVSV vectors to upregulate expression of HIV-1 antigens and coexpress molecular adjuvants are also being developed in an effort to balance immunogenicity and attenuation.
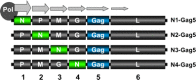
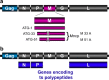
Figures





References
-
- Arnheiter H, Davis NL, Wertz G, et al. Role of the nucleocapsid protein in regulating vesicular stomatitis virus RNA synthesis. Cell. 1985;41:259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

