Intricate knots in proteins: Function and evolution
- PMID: 16978047
- PMCID: PMC1570178
- DOI: 10.1371/journal.pcbi.0020122
Intricate knots in proteins: Function and evolution
Abstract
Our investigation of knotted structures in the Protein Data Bank reveals the most complicated knot discovered to date. We suggest that the occurrence of this knot in a human ubiquitin hydrolase might be related to the role of the enzyme in protein degradation. While knots are usually preserved among homologues, we also identify an exception in a transcarbamylase. This allows us to exemplify the function of knots in proteins and to suggest how they may have been created.
Conflict of interest statement
Figures
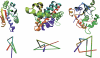
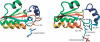
References
-
- Virnau P, Kantor Y, Kardar M. Knots in globule and coil phases of a model polyethylene. J Am Chem Soc. 2005;127:15102–15106. - PubMed
-
- Mansfield ML. Knots in Hamilton cycles. Macromolecules. 1994;27:5924–5926.
-
- Lua RC, Borovinskiy AL, Grosberg AY. Fractal and statistical properties of large compact polymers: A computational study. Polymer. 2004;45:717–731.
-
- Mansfield ML. Are there knots in proteins? Nat Struct Mol Bio. 1994;1:213–214. - PubMed
-
- Mansfield ML. Fit to be tied. Nat Struct Mol Bio. 1997;4:166–167. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

