M. leprae inhibits apoptosis in THP-1 cells by downregulation of Bad and Bak and upregulation of Mcl-1 gene expression
- PMID: 16978419
- PMCID: PMC1592106
- DOI: 10.1186/1471-2180-6-78
M. leprae inhibits apoptosis in THP-1 cells by downregulation of Bad and Bak and upregulation of Mcl-1 gene expression
Abstract
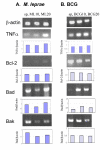
Background: Virulent Mycobacterium leprae interfere with host defense mechanisms such as cytokine activation and apoptosis. The mitochondrial pathway of apoptosis is regulated by the Bcl-2 family of proteins. Expression of Fas ligand and apoptotic proteins is found in leprosy lesions and M. leprae has been shown to activate pro-apoptotic Bcl-2 genes, Bak and Bax. However, the mechanism by which M. leprae modulates apoptosis is as yet unclear. We investigated expression of apoptotic genes in THP-1 monocytes in response to infection by M. leprae and non-pathogenic M. bovis BCG.
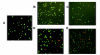
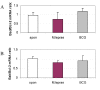
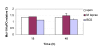
Results: M. leprae did not induce apoptosis in THP-1 cells, while BCG induced a significant loss of cell viability by 18 h post-infection at both (multiplicity of infection) MOI-10 and 20, with an increase by 48 h. BCG-induced cell death was accompanied by characteristic apoptotic DNA laddering in cells. Non-viable BCG had a limited effect on host cell death suggesting that BCG-induced apoptosis was a function of mycobacterial viability. M. leprae also activated lower levels of TNF-alpha secretion and TNF-alpha mRNA expression than BCG. Mycobacterium-induced activation of apoptotic gene expression was determined over a time course of infection. M. leprae reduced Bad and Bak mRNA expression by 18 h post-stimulation, with a further decrease at 48 h. Outcome of cell viability is determined by the ratio between pro- and anti-apoptotic proteins present in the cell. M. leprae infection resulted in downregulation of gene expression ratios, Bad/Bcl-2 mRNA by 39% and Bak/Bcl-2 mRNA by 23%. In contrast, live BCG increased Bad/Bcl-2 mRNA (29 %) but had a negligible effect on Bak/Bcl-2 mRNA. Heat killed BCG induced only a negligible (1-4 %) change in mRNA expression of either Bak/Bcl-2 or Bad/Bcl-2. Additionally, M. leprae upregulated the expression of anti-apoptotic gene Mcl-1 while, BCG downregulated Mcl-1 mRNA.
Conclusion: This study proposes an association between mycobacterium-induced apoptosis in THP-1 cells and the regulation of Bcl-2 family of proteins. M. leprae restricts apoptosis in THP-1 cells by downregulation of Bad and Bak and upregulation of Mcl-1 mRNA expression.
Figures


Similar articles
-
BCL-2 family expression in human neutrophils during delayed and accelerated apoptosis.J Leukoc Biol. 2001 Nov;70(5):783-92. J Leukoc Biol. 2001. PMID: 11698499
-
Experimental Mycobacterium leprae infection in BALB/c mice: effect of BCG administration on TNF-alpha production and granuloma development.Int J Lepr Other Mycobact Dis. 2000 Jun;68(2):156-66. Int J Lepr Other Mycobact Dis. 2000. PMID: 11036496
-
Extended survivability of prostate cancer cells in the absence of trophic factors: increased proliferation, evasion of apoptosis, and the role of apoptosis proteins.Cancer Res. 1998 Aug 1;58(15):3466-79. Cancer Res. 1998. PMID: 9699682
-
BAD: undertaker by night, candyman by day.Oncogene. 2008 Dec;27 Suppl 1:S53-70. doi: 10.1038/onc.2009.44. Oncogene. 2008. PMID: 19641507 Review.
-
Bcl-2 gene family and related proteins in mammary gland involution and breast cancer.J Mammary Gland Biol Neoplasia. 1999 Apr;4(2):153-64. doi: 10.1023/a:1018773123899. J Mammary Gland Biol Neoplasia. 1999. PMID: 10426394 Review.
Cited by
-
MicroRNA biomarkers in leprosy: insights from the Northern Brazilian Amazon population and their implications in disease immune-physiopathology.Front Genet. 2024 Jan 25;15:1320161. doi: 10.3389/fgene.2024.1320161. eCollection 2024. Front Genet. 2024. PMID: 38343694 Free PMC article.
-
Quercetin induces tongue squamous cell carcinoma cell apoptosis via the JNK activation-regulated ERK/GSK-3α/β-mediated mitochondria-dependent apoptotic signaling pathway.Oncol Lett. 2022 Mar;23(3):78. doi: 10.3892/ol.2022.13198. Epub 2022 Jan 11. Oncol Lett. 2022. PMID: 35111247 Free PMC article.
-
miRNome Expression Analysis Reveals New Players on Leprosy Immune Physiopathology.Front Immunol. 2018 Mar 9;9:463. doi: 10.3389/fimmu.2018.00463. eCollection 2018. Front Immunol. 2018. PMID: 29593724 Free PMC article. Clinical Trial.
-
PPARγ is critical for Mycobacterium tuberculosis induction of Mcl-1 and limitation of human macrophage apoptosis.PLoS Pathog. 2018 Jun 21;14(6):e1007100. doi: 10.1371/journal.ppat.1007100. eCollection 2018 Jun. PLoS Pathog. 2018. PMID: 29928066 Free PMC article.
-
Mcl-1 signals pathway inhibitors in mouse peritoneal macrophage apoptosis infected with the Xinjiang strain of M. tuberculosis.Int J Clin Exp Pathol. 2017 Dec 1;10(12):11952-11967. eCollection 2017. Int J Clin Exp Pathol. 2017. PMID: 31966560 Free PMC article.
References
-
- Perskvist N, Long M, Stendahl O, Zheng L. Mycobacterium tuberculosis promotes apoptosis in human neutrophils by activating caspase-3 and altering expression of Bax/Bcl-xL via an oxygen-dependent pathway. J Immunol. 2002;168:6358–6365. - PubMed
-
- Roach DR, Bean AGD, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–4627. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

