Foot-and-mouth disease virus 3C protease: recent structural and functional insights into an antiviral target
- PMID: 16979372
- PMCID: PMC7185863
- DOI: 10.1016/j.biocel.2006.07.006
Foot-and-mouth disease virus 3C protease: recent structural and functional insights into an antiviral target
Abstract
The 3C protease from foot-and-mouth disease virus (FMDV 3C(pro)) is critical for viral pathogenesis, having vital roles in both the processing of the polyprotein precursor and RNA replication. Although recent structural and functional studies have revealed new insights into the mechanism and function of the enzyme, key questions remain that must be addressed before the potential of FMDV 3C(pro) as an antiviral drug target can be realised.
Figures
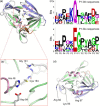
References
-
- Allaire M., Chernaia M.M., Malcolm B.A., James M.N. Picornaviral 3C cysteine proteinases have a fold similar to chymotrypsin-like serine proteinases. Nature. 1994;369(6475):72–76. - PubMed
-
- Barrett A.J., Rawlings N.D. Evolutionary lines of cysteine peptidases. Biol. Chem. 2001;382(5):727–733. - PubMed
-
- Bergmann E.M., Cherney M.M., McKendrick J., Frormann S., Luo C., Malcolm B.A. Crystal structure of an inhibitor complex of the 3C proteinase from hepatitis A virus (HAV) and implications for the polyprotein processing in HAV. Virology. 1999;265(1):153–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

